Back
Poster, Podium & Video Sessions
Podium
PD12: Bladder Cancer: Basic Research & Pathophysiology II
PD12-05: Spatial Transcriptomics Provides Evidence for Alternative Checkpoint Axes in BCG-treated NMIBC
Friday, May 13, 2022
1:40 PM – 1:50 PM
Location: Room 244
Daniel Ranti*, Yuanshuo Wang, Jorge Daza, Sanjana Shroff, Kristin Beaumont, Amir Horowitz, John Sfakianos, New York, NY

Daniel Ranti, BS
Icahn School of Medicine
Podium Presenter(s)
Introduction: Recurrence in BCG treated non-muscle invasive bladder cancer is significant. Disruption of the PD-1:PD-L1 axis via checkpoint inhibitors has shown promise, however studies show PD-L1 resistance may explain ~25% of recurrence. Antitumor immunity is critical in clearing recurrent tumors, and evidence supports HLA-E/NKG2A as a key checkpoint in NK clearance of tumors and CD8 T-cell infiltration. We used spatial sequencing (STseq) to visualize interactions between tumor, stroma, and immune cells, and to investigate the molecular signatures associated with NK and T cells interacting with tumor.
Methods: STseq was performed on a specimen from 1 BCG-resistant NMIBC case. Slides were processed and imaged according to the Visium 10X protocol. Cell lineages were defined by Robertson AG et al. (Cell. 2017). Visium spots with > 90th percentile of a linage signature were labelled as high-signature spots. Spots with multiple signatures, representing the plasticity across tumor lineages as well as the presence of NK or T cells, were annotated accordingly (Fig. 1A). We identified immune cells in and immediately surrounding tumor cells using NK, mutual NK and CD8 T cell, and tumor cell markers. Combined score of all listed genes and HLA-E were visualized (Fig. 1B). A differential gene expression analysis, scaled column-wise, was performed to understand the relationship between immune markers and tumor lineages (Fig. 1C).
Results: Neuronal tumor cells had the second highest observed co-expression of NK and CD8 T cell signatures and similarly expressed high levels of HLA-E. However, “neuronal” tumors lacked expression of PD-L1. We also observed NK and/or CD8 T cells infiltrating nests of other lineage subtypes, such as “p53”, but lacking activation signals. Lastly, preferential infiltration of “immune” tumor cell nests by NK and/or CD8 T cells was seen in areas of higher expression of HLA-E and CD274 (PD-L1).
Conclusions: These results suggest overlapping non-redundant mechanisms of evasion. Our analysis confirms preferential infiltration of immune tumor cell nests by NK and/or CD8 T cells where expression of HLA-E and PD-L1 is higher, and that interactions between tumor immune cells is heterogeneous within samples. This suggests that HLA-E could be a secondary inhibitory axis of BCG resistance via NK mediated HLA-E:NKG2A.
Source of Funding: NA
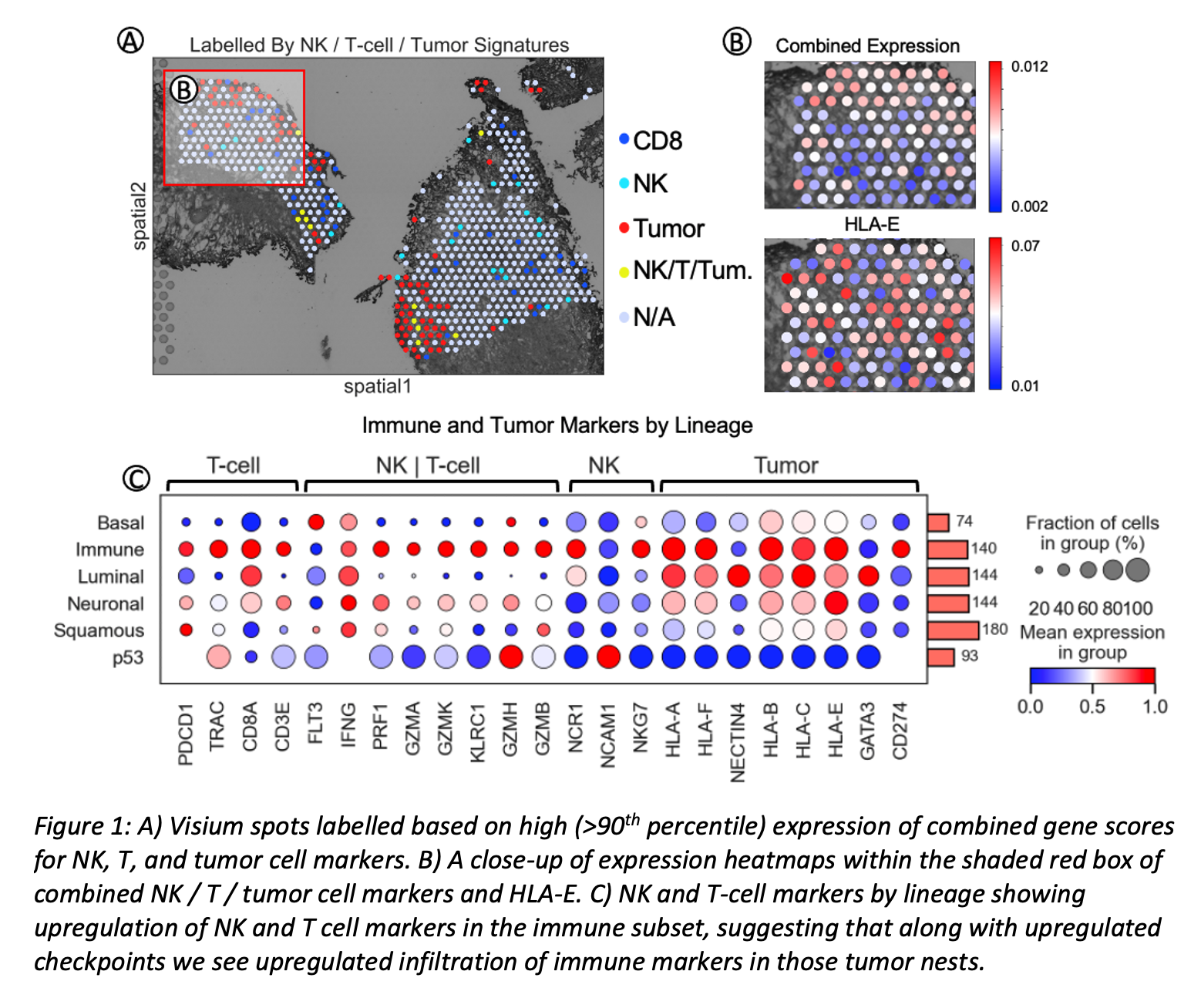
Methods: STseq was performed on a specimen from 1 BCG-resistant NMIBC case. Slides were processed and imaged according to the Visium 10X protocol. Cell lineages were defined by Robertson AG et al. (Cell. 2017). Visium spots with > 90th percentile of a linage signature were labelled as high-signature spots. Spots with multiple signatures, representing the plasticity across tumor lineages as well as the presence of NK or T cells, were annotated accordingly (Fig. 1A). We identified immune cells in and immediately surrounding tumor cells using NK, mutual NK and CD8 T cell, and tumor cell markers. Combined score of all listed genes and HLA-E were visualized (Fig. 1B). A differential gene expression analysis, scaled column-wise, was performed to understand the relationship between immune markers and tumor lineages (Fig. 1C).
Results: Neuronal tumor cells had the second highest observed co-expression of NK and CD8 T cell signatures and similarly expressed high levels of HLA-E. However, “neuronal” tumors lacked expression of PD-L1. We also observed NK and/or CD8 T cells infiltrating nests of other lineage subtypes, such as “p53”, but lacking activation signals. Lastly, preferential infiltration of “immune” tumor cell nests by NK and/or CD8 T cells was seen in areas of higher expression of HLA-E and CD274 (PD-L1).
Conclusions: These results suggest overlapping non-redundant mechanisms of evasion. Our analysis confirms preferential infiltration of immune tumor cell nests by NK and/or CD8 T cells where expression of HLA-E and PD-L1 is higher, and that interactions between tumor immune cells is heterogeneous within samples. This suggests that HLA-E could be a secondary inhibitory axis of BCG resistance via NK mediated HLA-E:NKG2A.
Source of Funding: NA


.jpg)
.jpg)