Back
Poster, Podium & Video Sessions
Podium
PD24: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Neurogenic Voiding Dysfunction
PD24-09: Initial training of an AI integrated intra urethral catheter pump prosthesis
Saturday, May 14, 2022
10:50 AM – 11:00 AM
Location: Room 245
William Hendricks, Sylvie M Kalikoff, Hannah A McKenney, Patricia Thai, Musa Ozturk, Alexis Hoyos, Alex Arevalos, Houston, TX, Kunj Sheth*, Palo Alto, CA

Kunj Sheth, MD, BS
Stanford University School of Medicine
Podium Presenter(s)
Introduction: The current standard of care for neurogenic bladder management is clean intermittent catheterization (CIC) 5-8 times daily. However, CIC is time consuming, carries a risk of urinary tract infection or urethral trauma, and ultimately leads to a diminished quality of life. A new class of devices, intraurethral catheter pumps, attempt to offer these patients a new alternative method to empty the bladder. Although showing a more favorable quality of life profile, these devices have had issues with tolerance due to their large size and lingering UTI risk. The current study presents benchtop testing of a novel sensor and AI integrated intraurethral pump prosthesis designed at harnessing the same benefits demonstrated by prior devices while offering additional comfort features and the earlier detection of pre-febrile symptomatic UTIs.
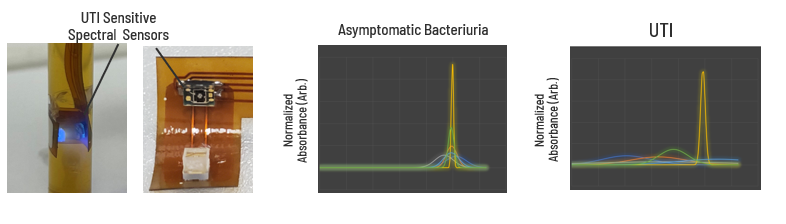
Methods: 17 Fr versions of the prototype prosthesis were assembled and tested on the benchtop, using human urine samples from chronic neurogenic catheter users known to be without a UTI and those suspected of a UTI. Multivariate logistic regression machine learning algorithms were implemented for the identification of an individual’s normal urine day to day variance and tracking changes from that baseline across multiple spectrum as a predictor of a UTI.
Results: In our preliminary results, 7 days of once a day urine sample sampled at the same approximate time was sufficient to capture day to day variance in an individual’s urine composition. The accuracy of the algorithm to then predict a UTI in a separate urine data set was found to have an R^2 value of 0.75 with a p-value <0.05 (n=420).
Conclusions: Our early results of our AI urine training studies are providing proof of principle evidence for our AI’s ability to statistically differentiate between an asymptomatic bacterial colonized bladder and a symptomatic urinary tract infection by tracking changes in specific spectral measurements over time. Such a device has the potential to vastly improve the quality of life of chronic catheter users by providing an alternative tech enabled bladder management tool that enables patients to pee at the push of a button and inform them and their doctor if they are developing a UTI before life threatening symptoms appear ultimately improving patient quality of life and lowering healthcare costs through hospitalization reduction.
Source of Funding: National Science Foundation 20-527 Small Business Innovation Research Program Phase I (12/01/2020 - 01/01/2022)
Methods: 17 Fr versions of the prototype prosthesis were assembled and tested on the benchtop, using human urine samples from chronic neurogenic catheter users known to be without a UTI and those suspected of a UTI. Multivariate logistic regression machine learning algorithms were implemented for the identification of an individual’s normal urine day to day variance and tracking changes from that baseline across multiple spectrum as a predictor of a UTI.
Results: In our preliminary results, 7 days of once a day urine sample sampled at the same approximate time was sufficient to capture day to day variance in an individual’s urine composition. The accuracy of the algorithm to then predict a UTI in a separate urine data set was found to have an R^2 value of 0.75 with a p-value <0.05 (n=420).
Conclusions: Our early results of our AI urine training studies are providing proof of principle evidence for our AI’s ability to statistically differentiate between an asymptomatic bacterial colonized bladder and a symptomatic urinary tract infection by tracking changes in specific spectral measurements over time. Such a device has the potential to vastly improve the quality of life of chronic catheter users by providing an alternative tech enabled bladder management tool that enables patients to pee at the push of a button and inform them and their doctor if they are developing a UTI before life threatening symptoms appear ultimately improving patient quality of life and lowering healthcare costs through hospitalization reduction.
Source of Funding: National Science Foundation 20-527 Small Business Innovation Research Program Phase I (12/01/2020 - 01/01/2022)

.jpg)
.jpg)