Back
Poster, Podium & Video Sessions
Podium
PD37: Surgical Technology & Simulation: Instrumentation & Technology II
PD37-12: Mutlicenter development and validation of a consensus based non-biohazardous Simulation Model for Holmium Laser Enucleation of the Prostate (HoLEP)
Sunday, May 15, 2022
8:50 AM – 9:00 AM
Location: Room 245
Rachel Melnyk, Christopher Wanderling, Rajat Jain, Scott Quarrier, Rochester, NY, Amy Krambeck, Chicago, IL, Nicole Miller, Nashville, TN, Gopal Narang, Mitchell Humphreys, Phoenix, AZ, Ahmed Ghazi*, Rochester, NY

Ahmed E. Ghazi, MD, FEBU, MBBS
University of Rochester
Podium Presenter(s)
Introduction: HoLEP is a an underutilized, minimally invasive approach for the management of benign prostatic hyperplasia. Its steep learning curve and lack of realistic simulation platforms with incorporated objective metrics has hindered the adoption of this technique. Our aim is to validate a high-fidelity non-biohazardous hydrogel platform fabricated to specifications attained through expert consensus.
Methods: Delphi methodology was utilized to gain consensus from an expert panel of endourologists. Overall, 250 questions addressed aspects to optimize a HoLEP simulation: overall utility, anatomical and procedural components, tissue fidelity, assessment of performance. Consensus (>80% agreement) over 3 rounds defined 81 essential elements that were incorporated into prototypes fabricated using a combination of hydrogel molding and 3D printing (Figure 1A). Validation was performed by comparing 6 experts and 6 novices performance from 3 centers using the consensus based objective and subjective metrics.
Results: The model contained all 9 anatomic components (ex. bladder, ureteric orifices, lateral and median lobes, capsule, urethra), 7 objective evaluation metrics (ex. injury of ureteric orifices or bladder neck, perforation of the bladder or prostate capsule), and 5 procedural steps except for hemostasis and morcellation. Categorically overall utility, anatomical and procedural components, tissue fidelity, and assessment of performance received an 82%, 78%, 73% and 82% satisfaction score by experts respectively. Laser and instrument tissue interactions achieved highest (>85%) satisfaction. Experts outperformed novices in enucleation time (15.1 vs 47.5 min p=0.001), resected adenoma weight (75.5 vs 49 g p=0.04) and developed global evaluation tool (27 vs 15.8 p=0.001) (Fig 1B). All experts agreed the model could provide a safe training alternative, be used to evaluate trainee performance, and trial new approaches in a risk-free environment.
Conclusions: This is the first consensus-based approach to design and initially validate of a non-biohazardous HoLEP simulation with incorporated evaluation metrics capable of supplementing HoLEP training. Further validation studies are required to establish its effectiveness as a training platform.
Source of Funding: NA
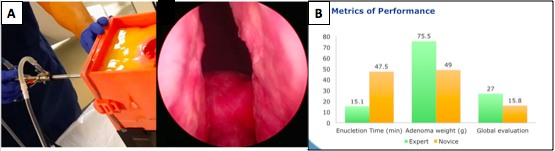
Methods: Delphi methodology was utilized to gain consensus from an expert panel of endourologists. Overall, 250 questions addressed aspects to optimize a HoLEP simulation: overall utility, anatomical and procedural components, tissue fidelity, assessment of performance. Consensus (>80% agreement) over 3 rounds defined 81 essential elements that were incorporated into prototypes fabricated using a combination of hydrogel molding and 3D printing (Figure 1A). Validation was performed by comparing 6 experts and 6 novices performance from 3 centers using the consensus based objective and subjective metrics.
Results: The model contained all 9 anatomic components (ex. bladder, ureteric orifices, lateral and median lobes, capsule, urethra), 7 objective evaluation metrics (ex. injury of ureteric orifices or bladder neck, perforation of the bladder or prostate capsule), and 5 procedural steps except for hemostasis and morcellation. Categorically overall utility, anatomical and procedural components, tissue fidelity, and assessment of performance received an 82%, 78%, 73% and 82% satisfaction score by experts respectively. Laser and instrument tissue interactions achieved highest (>85%) satisfaction. Experts outperformed novices in enucleation time (15.1 vs 47.5 min p=0.001), resected adenoma weight (75.5 vs 49 g p=0.04) and developed global evaluation tool (27 vs 15.8 p=0.001) (Fig 1B). All experts agreed the model could provide a safe training alternative, be used to evaluate trainee performance, and trial new approaches in a risk-free environment.
Conclusions: This is the first consensus-based approach to design and initially validate of a non-biohazardous HoLEP simulation with incorporated evaluation metrics capable of supplementing HoLEP training. Further validation studies are required to establish its effectiveness as a training platform.
Source of Funding: NA


.jpg)
.jpg)