Back
Poster, Podium & Video Sessions
Podium
PD52: Sexual Function/Dysfunction: Surgical Therapy II
PD52-03: Evaluation of Protection against Gram-positive and Gram-negative bacteria in a Novel In Vitro Biofilm Model of Penile Prosthesis
Monday, May 16, 2022
7:20 AM – 7:30 AM
Location: Room 252
Manish Narasimman*, Gregory Plano, Miami, FL, Jesse Ory, Halifax, Canada, Sara Schesser Bartra, Ranjith Ramasamy, Miami, FL

Manish Narasimman, BA
University of Miami
Podium Presenter(s)
Introduction: Late infection, thought to be due to biofilm formation, remains a feared complication after inflatable penile prosthesis (IPP) insertion. Our in vitro model previously showed S. epidermidis, S. aureus, P. aeruginosa, and K. pneumoniae can form significant biofilm on IPPs. P. aeruginosa showed the strongest predilection to grow biofilm. Understanding and preventing this biofilm formation could be a breakthrough in reducing IPP infections. We used our in vitro model to compare growth of different bacterial biofilms on IPPs with and without antibiotics and compare efficacy of antibiotic treated Coloplast Titan® and AMS 700 InhibiZone™ against biofilm formation.
Methods: Sterile IPPs were cut into rings. Coloplast rings were dipped in 10 ml of a 10 mg/ml Rifampin, 1 mg/ml Gentamicin, and deionized water solution and dried. Coloplast and AMS rings were incubated in S. epidermidis, S. aureus, P. aeruginosa, or K. pneumoniae cultures in tryptic soy broth (TSB) for a 2 h attachment period, followed by only TSB for 120 h. We utilized a published and validated method to measure biofilm mass. Rings were fixed with ethanol and biofilm formation measured by spectrophotometer (OD570) after crystal violet staining.
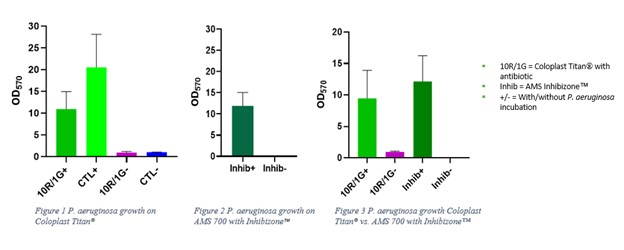
Results: Of the bacteria studied, only P. aeruginosa formed significant biofilm on antibiotic treated Coloplast Titan® and AMS 700 InhibiZone™ implant rings. In direct comparison of the implants, no significant difference was found in biofilm formation by P. aeruginosa.
Conclusions: Our novel in vitro model of biofilm formation of IPPs evaluated the effect of a gentamicin/rifampin antibiotic dip on Coloplast Titan® and the anti-infective capacity of minocycline/rifampin precoated AMS 700 InhibiZone™ against various bacteria. Our findings suggest P. aeruginosa can form a significant quantity of biofilm on IPPs even with antibiotic treatments. However, both treated IPPs appear to provide effective coverage against biofilm formation by S. epidermidis, S. aureus, and K. pneumoniae. No significant difference was found between the IPPs in protection against P. aeruginosa. P. aeruginosa should continue to be a specific target of investigation and antibiotic strategies potentially modified to increase coverage against the organism to reduce delayed post-operative IPP infections.
Source of Funding: This work was supported by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to Ranjith Ramasamy. This work was also supported by the AUA Summer Medical Student Fellowship Award to Manish Narasimman.
Methods: Sterile IPPs were cut into rings. Coloplast rings were dipped in 10 ml of a 10 mg/ml Rifampin, 1 mg/ml Gentamicin, and deionized water solution and dried. Coloplast and AMS rings were incubated in S. epidermidis, S. aureus, P. aeruginosa, or K. pneumoniae cultures in tryptic soy broth (TSB) for a 2 h attachment period, followed by only TSB for 120 h. We utilized a published and validated method to measure biofilm mass. Rings were fixed with ethanol and biofilm formation measured by spectrophotometer (OD570) after crystal violet staining.
Results: Of the bacteria studied, only P. aeruginosa formed significant biofilm on antibiotic treated Coloplast Titan® and AMS 700 InhibiZone™ implant rings. In direct comparison of the implants, no significant difference was found in biofilm formation by P. aeruginosa.
Conclusions: Our novel in vitro model of biofilm formation of IPPs evaluated the effect of a gentamicin/rifampin antibiotic dip on Coloplast Titan® and the anti-infective capacity of minocycline/rifampin precoated AMS 700 InhibiZone™ against various bacteria. Our findings suggest P. aeruginosa can form a significant quantity of biofilm on IPPs even with antibiotic treatments. However, both treated IPPs appear to provide effective coverage against biofilm formation by S. epidermidis, S. aureus, and K. pneumoniae. No significant difference was found between the IPPs in protection against P. aeruginosa. P. aeruginosa should continue to be a specific target of investigation and antibiotic strategies potentially modified to increase coverage against the organism to reduce delayed post-operative IPP infections.
Source of Funding: This work was supported by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to Ranjith Ramasamy. This work was also supported by the AUA Summer Medical Student Fellowship Award to Manish Narasimman.


.jpg)
.jpg)