Back
Poster, Podium & Video Sessions
Best Poster Award
MP02: Infections/Inflammation/Cystic Disease of the Genitourinary Tract: Kidney & Bladder I
MP02-15: Impact of MV140 on patient related burden of disease associated with the management of recurrent urinary tract infections (rUTI)
Friday, May 13, 2022
7:00 AM – 8:15 AM
Location: Room 225
J. Curtis Nickel*, Kingston, Canada, Stephen Foley, Reading, United Kingdom, Miguel Casanovas, Raquel Caballero, Madrid, Spain, María Fernanda Lorenzo-Gómez, Salamanca, Spain

Curtis J. Nickel, MD, FRCSC (he/him/his)
Professor of Urology
Queen's University at Kingston Canada
Poster Presenter(s)
Introduction: Women with rUTI suffer from episodic pain and urinary symptoms, disability, costs, poor quality of life, as well as short- and long-term complications of antibiotic therapy. A randomized placebo-controlled trial evaluating a polybacterial sublingual vaccine, MV140, presented as a late breaking AUA abstract in September 2021 showed efficacy and safety in reducing the risk of developing UTI in women with rUTI (ITT n= 215). The personal impact of this vaccine on burden of disease in the women enrolled in this study was evaluated.
Methods: Female subjects with rUTI were randomized to placebo for 6 months or vaccine for 3 or 6 months (1:1:1 ratio) and followed for 12 months from initiation of therapy. The primary endpoint and major secondary endpoint were the mean number of UTI and UTI free rate in the 9-month efficacy period following 3 months of intervention. Secondary analyses relevant to this analysis included impact on patient safety, symptom severity, antibiotic use, healthcare utilization, costs and quality of life.
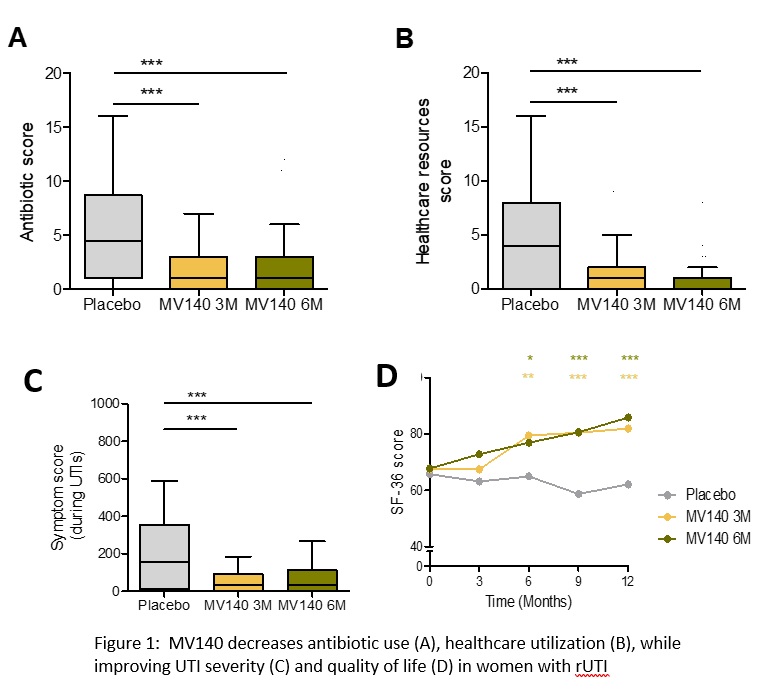
Results: As previously reported, there was a significant reduction in UTI episodes (3.0 in placebo group [IQR, 0.5-6.0] compared to 0.0 [IQR, 0.0-1.0] in both groups receiving MV140 [P <0.001]) and UTI-free rate (25.0% in placebo, 55.7% in MV140 3M and 58.0% in MV140 6M groups; P<0.001) with few treatment-related adverse events in all groups (study total n=9). The median number of antibiotic prescriptions was 4.5 [IQR, 1.0-8.5] for placebo group compared to 1.0 [IQR, 0.0-3.0] in both groups receiving MV140 (P <0.001). A significant reduction (75.0-100.0% decrease) in the need for healthcare resources with similar reduction in healthcare costs were observed in the vaccinated subjects. UTI symptom severity was significantly reduced in the vaccinated groups with a concomitant improvement in quality of life experienced by the vaccinated subjects compared to those receiving placebo (Figure 1).
Conclusions: MV140 significantly reduces the personal burden of UTI disease by safely decreasing risk of subsequent UTI, reducing severity of breakthrough UTI, reducing antibiotic use, healthcare resources, costs and ultimately improving the quality of life in women suffering from rUTI.
Source of Funding: Inmunotek
Methods: Female subjects with rUTI were randomized to placebo for 6 months or vaccine for 3 or 6 months (1:1:1 ratio) and followed for 12 months from initiation of therapy. The primary endpoint and major secondary endpoint were the mean number of UTI and UTI free rate in the 9-month efficacy period following 3 months of intervention. Secondary analyses relevant to this analysis included impact on patient safety, symptom severity, antibiotic use, healthcare utilization, costs and quality of life.
Results: As previously reported, there was a significant reduction in UTI episodes (3.0 in placebo group [IQR, 0.5-6.0] compared to 0.0 [IQR, 0.0-1.0] in both groups receiving MV140 [P <0.001]) and UTI-free rate (25.0% in placebo, 55.7% in MV140 3M and 58.0% in MV140 6M groups; P<0.001) with few treatment-related adverse events in all groups (study total n=9). The median number of antibiotic prescriptions was 4.5 [IQR, 1.0-8.5] for placebo group compared to 1.0 [IQR, 0.0-3.0] in both groups receiving MV140 (P <0.001). A significant reduction (75.0-100.0% decrease) in the need for healthcare resources with similar reduction in healthcare costs were observed in the vaccinated subjects. UTI symptom severity was significantly reduced in the vaccinated groups with a concomitant improvement in quality of life experienced by the vaccinated subjects compared to those receiving placebo (Figure 1).
Conclusions: MV140 significantly reduces the personal burden of UTI disease by safely decreasing risk of subsequent UTI, reducing severity of breakthrough UTI, reducing antibiotic use, healthcare resources, costs and ultimately improving the quality of life in women suffering from rUTI.
Source of Funding: Inmunotek


.jpg)
.jpg)