Back
Poster, Podium & Video Sessions
MP03: Bladder Cancer: Invasive I
MP03-14: Neoadjuvant versus adjuvant chemotherapy in bladder cancer: A nationwide cohort study
Friday, May 13, 2022
7:00 AM – 8:15 AM
Location: Room 222
Se Young Choi, Taekyu Huh, Joonhee Gook, Jae Hun Shim*, Jong Hyun Tae, Byung Hoon Chi, Jin Wook Kim, In Ho Chang, Tae-Hyoung Kim, Soon Chul Myung, Seoul, Korea, Republic of
Poster Presenter(s)
Introduction:
Background: Neoadjuvant chemotherapy (NAC) could improve overall survival (5-8% at 5-years) and is recommended for muscle-invasive bladder cancer in many guidelines. Despite a high level of evidence, NAC has not been used widely in real world. We tried to survey the trends in NAC and adjuvant chemotherapy (AC) use and compared overall survivals in nationwide population-based data.
Methods:
Methods: We collected the National Health Insurance Service database with radical cystectomy (RC) by bladder cancer from 2004 to 2016. We excluded other cancer diagnosis within 2 years, simultaneous neoadjuvant and adjuvant chemotherapy and incorrect information. We evaluated overall treatment trends. NAC and AC group were matched by propensity score. Cox proportional hazard analysis and Kaplan-Meier analysis were used.
Results:
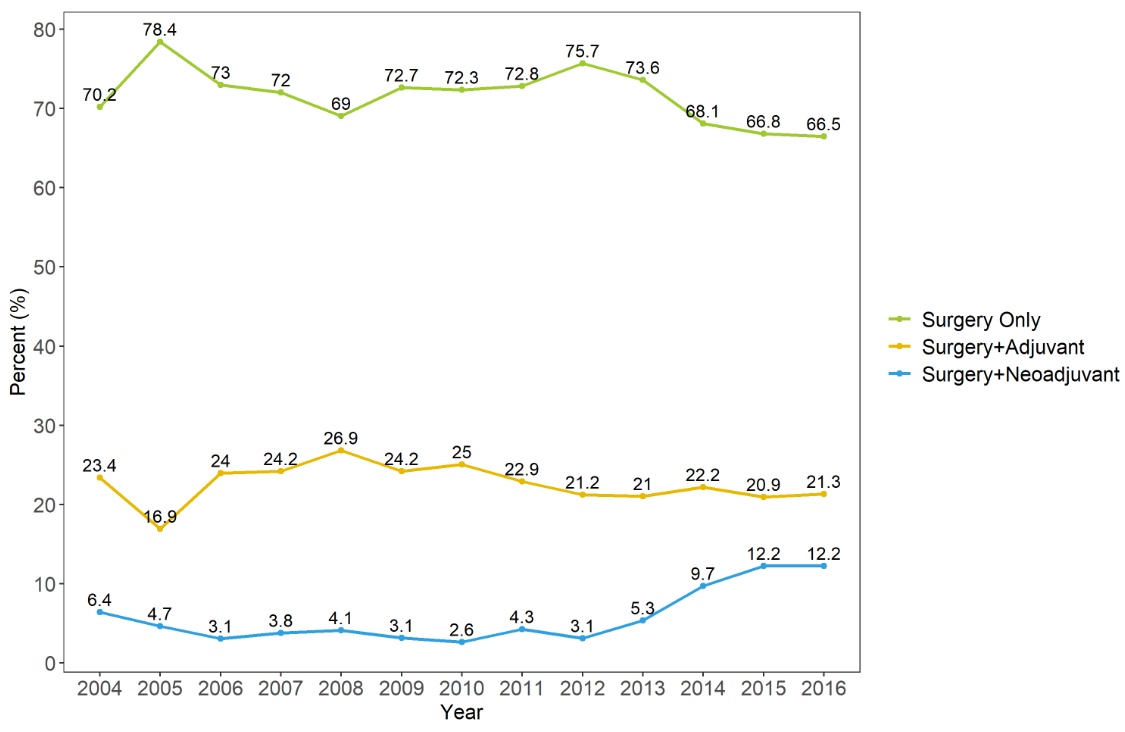
Results: Among 6,134 patients, there were 4,366 patients (71.2%) of only RX, 1,379 patients (22.5%) of AC, and 389 patients (6.3%). In relatively recent years, NAC increased to about 10%. Usage of granulocyte colony stimulating factor (GCSF) was lower in NAC than AC (19.0% vs 33.2%, p<0.001). The used number of GCSF was lower in NAC than AC (1.9 ± 1.9 vs 2.5 ± 2.6, p=0.027). After the propensity score matching, NAC showed significantly better overall survival (OS) than AC (p=0.004). In multivariate analysis, NAC was associated with better OS (hazard ratio 0.77, 95% confidence interval 0.65-0.92, p=0.003).
Conclusions:
Conclusion: Although NAC increased in relatively recent years, the percent of NAC was still low. In nationwide cohort, patients with NAC showed lower GCSF usage and better OS than AC. Expansion of NAC could give some benefits to patients with RC.
Source of Funding: This research was supported by the Korean Urological Oncology Society Grants (KUOS 21-06).
Background: Neoadjuvant chemotherapy (NAC) could improve overall survival (5-8% at 5-years) and is recommended for muscle-invasive bladder cancer in many guidelines. Despite a high level of evidence, NAC has not been used widely in real world. We tried to survey the trends in NAC and adjuvant chemotherapy (AC) use and compared overall survivals in nationwide population-based data.
Methods:
Methods: We collected the National Health Insurance Service database with radical cystectomy (RC) by bladder cancer from 2004 to 2016. We excluded other cancer diagnosis within 2 years, simultaneous neoadjuvant and adjuvant chemotherapy and incorrect information. We evaluated overall treatment trends. NAC and AC group were matched by propensity score. Cox proportional hazard analysis and Kaplan-Meier analysis were used.
Results:
Results: Among 6,134 patients, there were 4,366 patients (71.2%) of only RX, 1,379 patients (22.5%) of AC, and 389 patients (6.3%). In relatively recent years, NAC increased to about 10%. Usage of granulocyte colony stimulating factor (GCSF) was lower in NAC than AC (19.0% vs 33.2%, p<0.001). The used number of GCSF was lower in NAC than AC (1.9 ± 1.9 vs 2.5 ± 2.6, p=0.027). After the propensity score matching, NAC showed significantly better overall survival (OS) than AC (p=0.004). In multivariate analysis, NAC was associated with better OS (hazard ratio 0.77, 95% confidence interval 0.65-0.92, p=0.003).
Conclusions:
Conclusion: Although NAC increased in relatively recent years, the percent of NAC was still low. In nationwide cohort, patients with NAC showed lower GCSF usage and better OS than AC. Expansion of NAC could give some benefits to patients with RC.
Source of Funding: This research was supported by the Korean Urological Oncology Society Grants (KUOS 21-06).


.jpg)
.jpg)
.jpg)