Back
Poster, Podium & Video Sessions
Moderated Poster
MP05: Stone Disease: Basic Research & Pathophysiology
MP05-13: Impact of Ureteral Stents on Factors Contributing to Stricture Pathogenesis
Friday, May 13, 2022
8:45 AM – 10:00 AM
Location: Room 225
Karen Doersch*, Rochester, NY, Khaled Almutairi, Ben Chew, Dirk Lange, Vancouver, Canada
- KD
Karen M. Doersch, MD, PHD
Resident Physician
University of Rochester Medical Center
Poster Presenter(s)
Introduction: Ureteral stenting is common for managing obstructive uropathy. This study seeks to evaluate immunoinflammatory changes in the stented ureter and determine whether stent-related inflammation in the ureter could contribute to ureteral dysfunction.
Methods: Pigs were stented unilaterally for 14 days (stented group) and compared to the contralateral unstented ureter (unstented group). Additional pig ureters were stented for 14 days and then allowed to recover for 7 days (recovered group). Ureters from pigs that were never stented were also evaluated (control group). N=3 for all groups. Ureters were analyzed by RNAseq and proteomic analysis to evaluate the presence of pro-fibrotic factors.
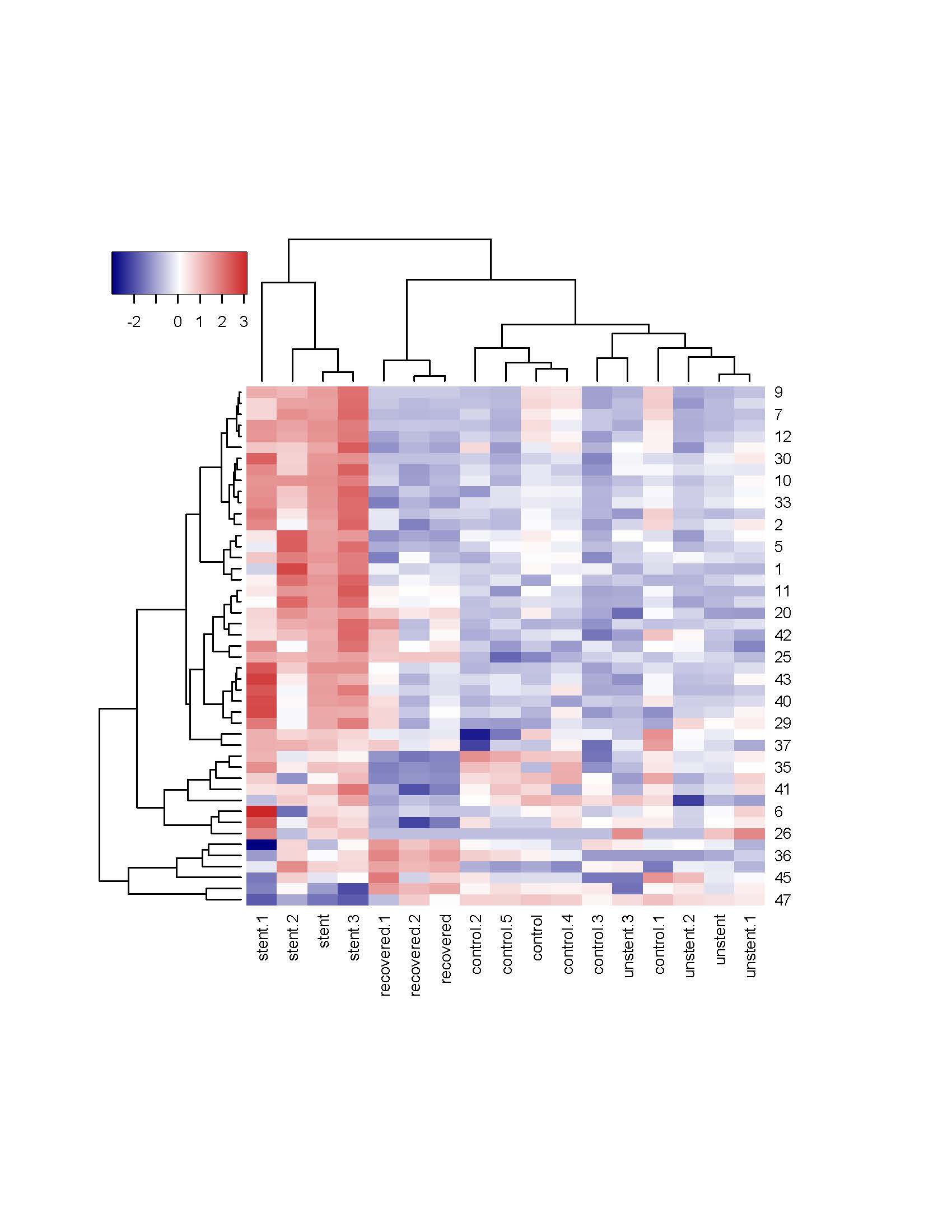
Results: RNAseq demonstrated clustering of the unstented, stented, and recovered groups separately on hierarchical clustering with the control group and unstented group segregating together (Figure 1). The stented ureter had increased transforming growth factor (TGF)-ß2, and TGF-ß-receptor(R)-2 and multiple collagens (Col) RNA levels compared to the unstented group. Compared to the stented group, the recovered group had alterations in TGF-ß2 and Col 4A2, 6A5, 1A2, and 3A1 RNA expression. Compared to the unstented group, the recovered group had increased expression of Col 12A1, 17A1 and 8A1 mRNA and decreased Col 53A and 8A2 mRNA. Proteomic analysis demonstrated that compared to the unstented group, the recovered group had increased Col 6A2, 6A3, and 7A1 and decreased Col 6A6 and 5A3. The recovered group had few differences in protein expression and no changes in Col levels compared to the stented group, indicating that many of the changes are recoverable.
Conclusions: Indwelling stents result in alterations in signaling pathways associated with fibrosis formation, which may contribute to stent-associated ureteral dysfunction. Many of these alterations improve 7 days following stent removal, but some aberrancies remain. These changes do not appear to affect contralateral unstented ureters. More work is needed to fully characterize pro-fibrotic changes in stented ureters, which may help elucidate causes and treatment strategies for stent-related ureteral dysfunction, pain, and possibly stricture pathogenesis.
Source of Funding: Boston Scientific Investigator Initiated Research Grant to Dirk Lange
Methods: Pigs were stented unilaterally for 14 days (stented group) and compared to the contralateral unstented ureter (unstented group). Additional pig ureters were stented for 14 days and then allowed to recover for 7 days (recovered group). Ureters from pigs that were never stented were also evaluated (control group). N=3 for all groups. Ureters were analyzed by RNAseq and proteomic analysis to evaluate the presence of pro-fibrotic factors.
Results: RNAseq demonstrated clustering of the unstented, stented, and recovered groups separately on hierarchical clustering with the control group and unstented group segregating together (Figure 1). The stented ureter had increased transforming growth factor (TGF)-ß2, and TGF-ß-receptor(R)-2 and multiple collagens (Col) RNA levels compared to the unstented group. Compared to the stented group, the recovered group had alterations in TGF-ß2 and Col 4A2, 6A5, 1A2, and 3A1 RNA expression. Compared to the unstented group, the recovered group had increased expression of Col 12A1, 17A1 and 8A1 mRNA and decreased Col 53A and 8A2 mRNA. Proteomic analysis demonstrated that compared to the unstented group, the recovered group had increased Col 6A2, 6A3, and 7A1 and decreased Col 6A6 and 5A3. The recovered group had few differences in protein expression and no changes in Col levels compared to the stented group, indicating that many of the changes are recoverable.
Conclusions: Indwelling stents result in alterations in signaling pathways associated with fibrosis formation, which may contribute to stent-associated ureteral dysfunction. Many of these alterations improve 7 days following stent removal, but some aberrancies remain. These changes do not appear to affect contralateral unstented ureters. More work is needed to fully characterize pro-fibrotic changes in stented ureters, which may help elucidate causes and treatment strategies for stent-related ureteral dysfunction, pain, and possibly stricture pathogenesis.
Source of Funding: Boston Scientific Investigator Initiated Research Grant to Dirk Lange


.jpg)
.jpg)