Back
Poster, Podium & Video Sessions
Moderated Poster
MP05: Stone Disease: Basic Research & Pathophysiology
MP05-15: The Gulo(-/-) Mouse: A Useful Model for Studying Endogenous Oxalate Production From Ascorbic Acid Turnover
Friday, May 13, 2022
8:45 AM – 10:00 AM
Location: Room 225
Joseph Crivelli*, Xingsheng Li, Sonia Fargue, Kyle Wood, Dean Assimos, Ross Holmes, John Knight, Birmingham, AL

Joseph J. Crivelli, MD
University of Alabama at Birmingham School of Medicine
Poster Presenter(s)
Introduction: At least half of the urinary oxalate pool is derived from endogenous production, to which turnover of ascorbic acid (AA) is a major contributor. The Gulo(-/-) mouse is an ideal model for the study of AA metabolism because, like humans, it cannot synthesize AA. We examined the contribution of AA to urinary oxalate excretion in Gulo(-/-) mice.
Methods: Eight Gulo(-/-) mice (4 male and 4 female) on an ultra-low oxalate diet underwent implantation of an osmotic pump following deprivation of dietary AA for 5 days. The osmotic pump infused 10 µmol/kg/h carbon-13-labeled AA (13C6-AA). On days 9-13 after implantation, plasma 13C6-AA and 24-hour urine 13C2-oxalate measurements were performed using high-performance liquid chromatography and ion chromatography coupled with mass spectrometry. The contribution of AA to urinary oxalate excretion was calculated as: [24-hour urine 13C2-oxalate % mole enrichment / Plasma 13C6-AA % mole enrichment] x 100. Brain, liver, kidney, and muscle were obtained at necropsy and immediately stored in liquid nitrogen for measurement of AA.
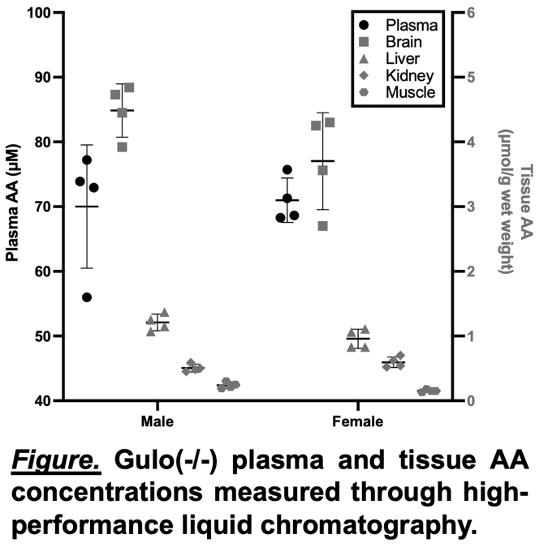
Results: The isotopic purity of 13C6-AA was 98%, and plasma and tissue 13C6-AA mole enrichments consistently exceeded 95%. The mean contribution of AA to urinary oxalate excretion was 25.1% ± 2.9%, which was similar between male and female mice (24.2% ± 2.3% and 26.0% ± 3.4%, respectively). Consistent concentrations of AA in plasma and tissue were observed between animals and by sex (Figure).
Conclusions: In Gulo(-/-) mice, one quarter of the urinary oxalate pool was derived from AA infused via an osmotic pump. Reproducible concentrations of plasma and tissue AA were achieved in this setting. Further utilization of this model will add to our knowledge of endogenous oxalate synthesis.
Source of Funding: Urology Care Foundation Research Scholar/Endourological Society/Raju Thomas, MD Award (JC), NIH 1R01DK126774 (RH and JK), 5K08DK115833 (KW), and 5P20DK119788 (DA).
Methods: Eight Gulo(-/-) mice (4 male and 4 female) on an ultra-low oxalate diet underwent implantation of an osmotic pump following deprivation of dietary AA for 5 days. The osmotic pump infused 10 µmol/kg/h carbon-13-labeled AA (13C6-AA). On days 9-13 after implantation, plasma 13C6-AA and 24-hour urine 13C2-oxalate measurements were performed using high-performance liquid chromatography and ion chromatography coupled with mass spectrometry. The contribution of AA to urinary oxalate excretion was calculated as: [24-hour urine 13C2-oxalate % mole enrichment / Plasma 13C6-AA % mole enrichment] x 100. Brain, liver, kidney, and muscle were obtained at necropsy and immediately stored in liquid nitrogen for measurement of AA.
Results: The isotopic purity of 13C6-AA was 98%, and plasma and tissue 13C6-AA mole enrichments consistently exceeded 95%. The mean contribution of AA to urinary oxalate excretion was 25.1% ± 2.9%, which was similar between male and female mice (24.2% ± 2.3% and 26.0% ± 3.4%, respectively). Consistent concentrations of AA in plasma and tissue were observed between animals and by sex (Figure).
Conclusions: In Gulo(-/-) mice, one quarter of the urinary oxalate pool was derived from AA infused via an osmotic pump. Reproducible concentrations of plasma and tissue AA were achieved in this setting. Further utilization of this model will add to our knowledge of endogenous oxalate synthesis.
Source of Funding: Urology Care Foundation Research Scholar/Endourological Society/Raju Thomas, MD Award (JC), NIH 1R01DK126774 (RH and JK), 5K08DK115833 (KW), and 5P20DK119788 (DA).


.jpg)
.jpg)