Back
Poster, Podium & Video Sessions
Moderated Poster
MP06: Bladder Cancer: Basic Research & Pathophysiology I
MP06-10: KDM6A deficiency in urothelium produces a distinct immune phenotype in a mouse model of bladder cancer
Friday, May 13, 2022
8:45 AM – 10:00 AM
Location: Room 222
Ao Zhang*, Hong Qiu, Timothy Chan, Byron Lee, Cleveland, OH
- AZ
Ao Zhang, MD, PHD
Cleveland Clinic
Poster Presenter(s)
Introduction: Lysine-specific demethylase 6A (KDM6A) is a chromatin modifier that is frequently mutated in bladder cancer and causes epigenetic dysregulation. Recently immune check point inhibition has shown great clinical promise in bladder cancer. Because epigenetic signature has been shown to predict response to immunotherapy, here we assessed if loss of KDM6A leads to alterations of tumor immune microenvironment in a mouse bladder cancer model.
Methods: Kdm6a urothelium conditional knockout (cKO) mice were generated by crossing Kdm6aflox/flox mice with Upk3aCre mice that express Cre recombinase in the urothelium. Littermate control mice were of the same genetic background but intact Kdm6a gene. Mice at 8 weeks of age (three in each group) were fed with drinking water containing 0.05% N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN). Bladders were harvested after 4 weeks and analyzed with H&E staining. Single cell RNA sequencing was performed on urothelium from control and Kdm6A cKO mice without BBN treatment.
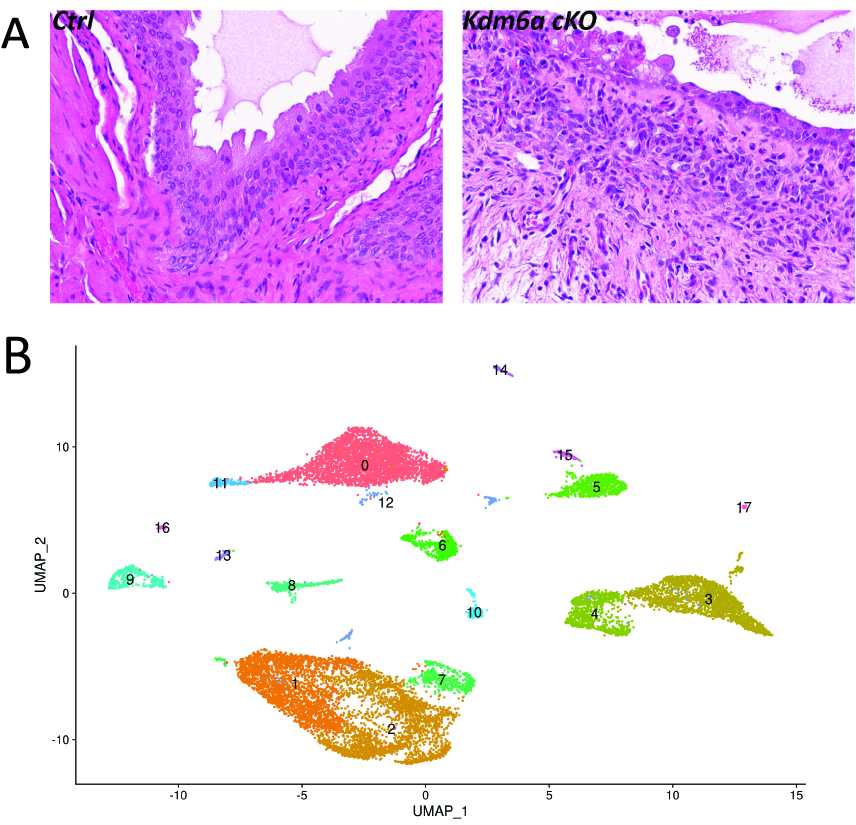
Results: After treatment with BBN for 4 weeks, there was marked edema and inflammatory changes with increased infiltration of immune cells in H&E stained bladder sections in Kdm6a cKO mice compared to Kdm6a intact controls (Figure A). While urothelium from control mice maintained grossly unchanged tissue architecture, urothelium from cKO mice exhibited cytologic atypia with loss of organized cell layer. Comparison of single cell RNA sequencing profile revealed enrichment of immune cell population in cKO mice (Figure B, cluster 12), which was consistent with histological findings.
Conclusions: Loss of KDM6A in the urothelium creates a distinct immune microenvironment in the BBN mouse bladder cancer model. Whether this immune cell-rich microenvironment affects response to immune check point inhibition warrants further investigation.
Source of Funding: This work was supported in part by the 2021 Urology Care Foundation Residency Research Award Program and the Kahlert Foundation.
Methods: Kdm6a urothelium conditional knockout (cKO) mice were generated by crossing Kdm6aflox/flox mice with Upk3aCre mice that express Cre recombinase in the urothelium. Littermate control mice were of the same genetic background but intact Kdm6a gene. Mice at 8 weeks of age (three in each group) were fed with drinking water containing 0.05% N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN). Bladders were harvested after 4 weeks and analyzed with H&E staining. Single cell RNA sequencing was performed on urothelium from control and Kdm6A cKO mice without BBN treatment.
Results: After treatment with BBN for 4 weeks, there was marked edema and inflammatory changes with increased infiltration of immune cells in H&E stained bladder sections in Kdm6a cKO mice compared to Kdm6a intact controls (Figure A). While urothelium from control mice maintained grossly unchanged tissue architecture, urothelium from cKO mice exhibited cytologic atypia with loss of organized cell layer. Comparison of single cell RNA sequencing profile revealed enrichment of immune cell population in cKO mice (Figure B, cluster 12), which was consistent with histological findings.
Conclusions: Loss of KDM6A in the urothelium creates a distinct immune microenvironment in the BBN mouse bladder cancer model. Whether this immune cell-rich microenvironment affects response to immune check point inhibition warrants further investigation.
Source of Funding: This work was supported in part by the 2021 Urology Care Foundation Residency Research Award Program and the Kahlert Foundation.


.jpg)
.jpg)