Back
Poster, Podium & Video Sessions
Moderated Poster
MP06: Bladder Cancer: Basic Research & Pathophysiology I
MP06-15: The Long Arm of Chromosome 9 Contains a Gene That Regulates PPAR&[gamma] in Bladder Cancer
Friday, May 13, 2022
8:45 AM – 10:00 AM
Location: Room 222
Ryutaro Shimizu*, Masashi Honda, Shogo Teraoka, Tetsuya Yumioka, Bunya Kawamoto, Noriya Yamaguchi, Hideto Iwamoto, Panagiota Tsounapi, Shuichi Morizane, Katsuya Hikita, Takahito Ohira, Hiroyuki Kugoh, Atsushi Takenaka, Yonago, Japan
- RS
Poster Presenter(s)
Introduction: Bladder cancers are roughly two molecular subtypes: luminal and basal, forming papillary and nodular tumors, respectively. In the early development of both types, high frequency of loss of heterozygosity (LOH) on the long arm of human chromosome 9 (9q) is observed, suggesting the presence of an important gene (group) on 9q involved in bladder cancer development. Here, we investigated the effects of chromosome 9q transfer on the cancer phenotype and molecular subtypes to elucidate the carcinogenic process in bladder cancer (Fig.1).
Methods: The basal-type bladder cancer cell line SCaBER was introduced with 9q (SCaBER#9q) via microcell-mediated chromosome transfer (MMCT) (Fig.2), using chromosome 4 (SCaBER#4) as a control. Proliferative and migrative capability changes were analyzed in each microcell hybrid clone by cell proliferation assay and wound healing assay. The expression levels of genetic markers of the luminal type (FOXA1, GATA3, and PPAR?) were examined by qRT-PCR and Western blotting.
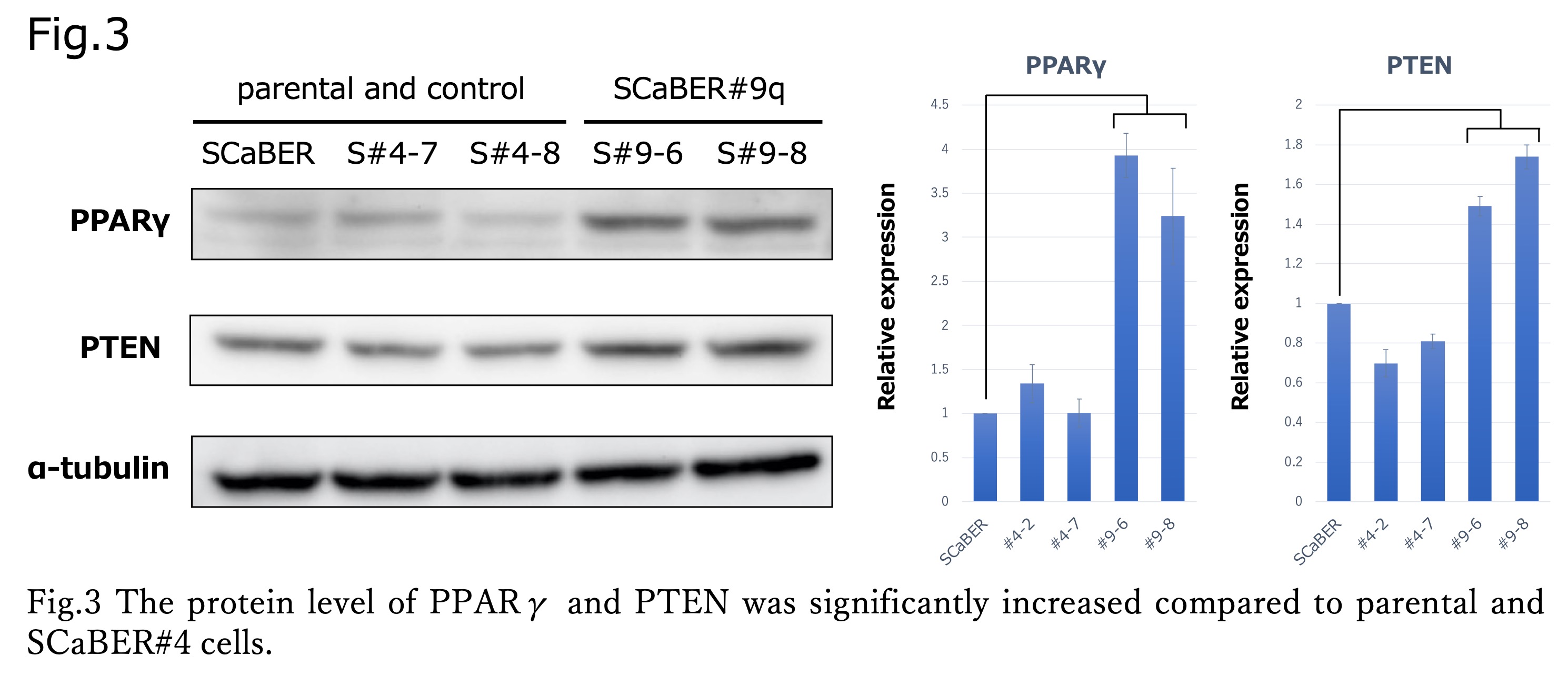
Results: SCaBER#9q cells presented with significant morphological changes characterized by an enlarged and somewhat flat shape compared to parental SCaBER cells and SCaBER#4 cells. Cell proliferation assay showed a significant decrease in proliferative capacity of SCaBER#9q compared to the parental strain and control (p < 0.001) (Fig.3 left). Wound-healing assay also showed a significant decrease in migration ability of SCaBER#9q (p < 0.001). SCaBER#9q cells showed increased expression of FOXA1, GATA3, and PPAR?, but Western blotting analysis showed that only PPAR? expression was significantly upregulated (3.0-4.4-fold).
Conclusions: Transfer of 9q into SCaBER cells increased the expression of PPAR? and decreased cell proliferation and migration, suggesting the presence of a group of genes regulating PPAR? in the 9q region.
Source of Funding: nothing
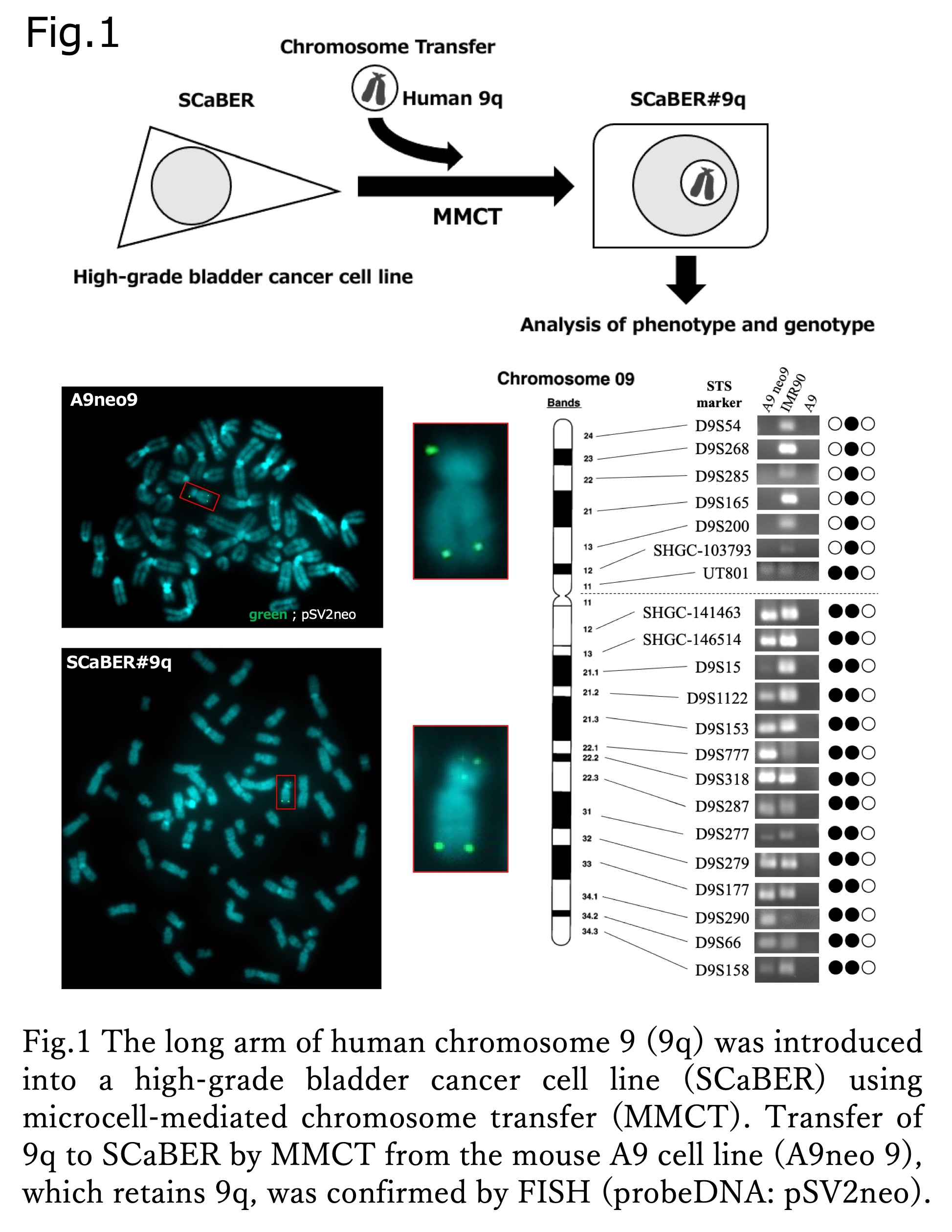
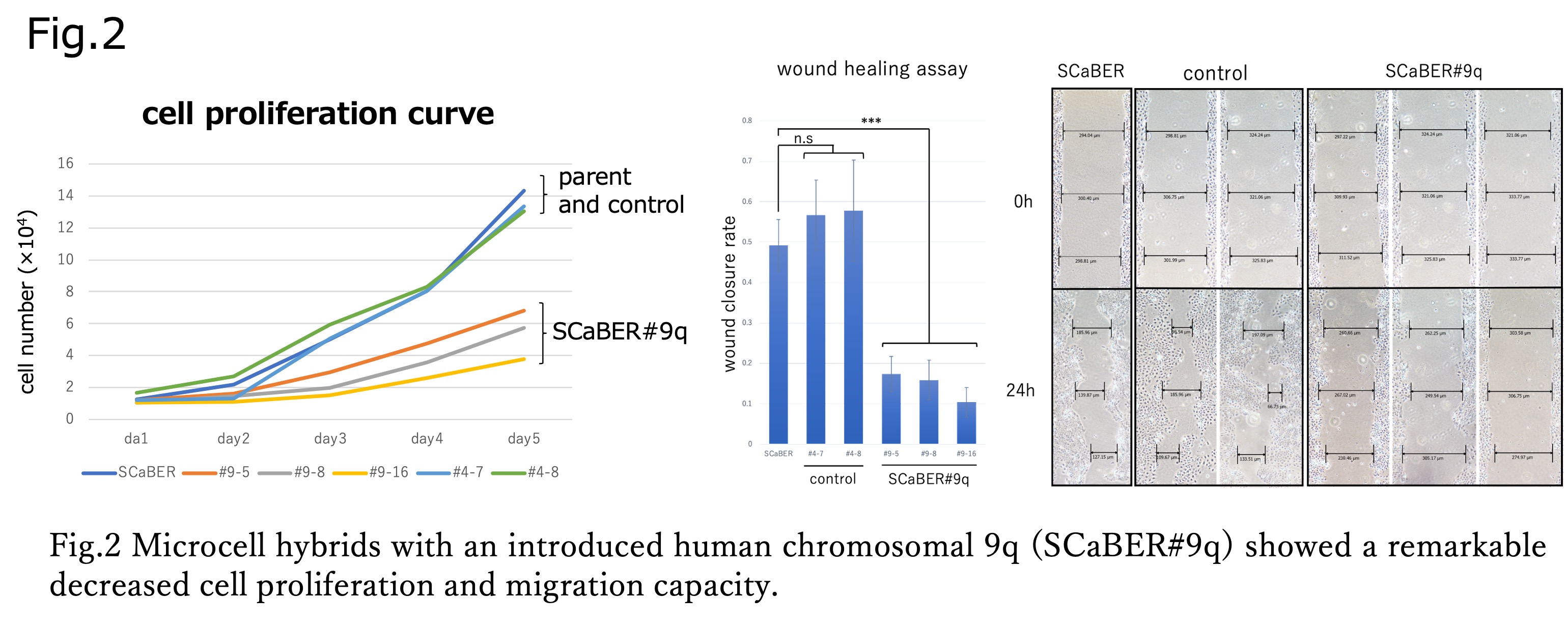
Methods: The basal-type bladder cancer cell line SCaBER was introduced with 9q (SCaBER#9q) via microcell-mediated chromosome transfer (MMCT) (Fig.2), using chromosome 4 (SCaBER#4) as a control. Proliferative and migrative capability changes were analyzed in each microcell hybrid clone by cell proliferation assay and wound healing assay. The expression levels of genetic markers of the luminal type (FOXA1, GATA3, and PPAR?) were examined by qRT-PCR and Western blotting.
Results: SCaBER#9q cells presented with significant morphological changes characterized by an enlarged and somewhat flat shape compared to parental SCaBER cells and SCaBER#4 cells. Cell proliferation assay showed a significant decrease in proliferative capacity of SCaBER#9q compared to the parental strain and control (p < 0.001) (Fig.3 left). Wound-healing assay also showed a significant decrease in migration ability of SCaBER#9q (p < 0.001). SCaBER#9q cells showed increased expression of FOXA1, GATA3, and PPAR?, but Western blotting analysis showed that only PPAR? expression was significantly upregulated (3.0-4.4-fold).
Conclusions: Transfer of 9q into SCaBER cells increased the expression of PPAR? and decreased cell proliferation and migration, suggesting the presence of a group of genes regulating PPAR? in the 9q region.
Source of Funding: nothing




.jpg)
.jpg)