Back
Poster, Podium & Video Sessions
Moderated Poster
MP07: Bladder & Urethra: Anatomy, Physiology & Pharmacology
MP07-05: Bladder oxidative stress and ERK signaling contribute to bladder pain mediated by macrophage migration inhibitory factor and High Mobility Group Box 1
Friday, May 13, 2022
10:30 AM – 11:45 AM
Location: Room 228
Shaojing Ye, Fei Ma, Dlovan Mahmood, Lexington, KY, Katherine Meyer-Siegler, St Petersburg, FL, Lin Leng, Richard Bucala, New Haven, CT, Pedro Vera*, Lexington, KY
- PV
Pedro L. Vera, PhD
University of Kentucky
Poster Presenter(s)
Introduction: Intravesical PAR4 induced bladder hyperalgesia (BHA) in mice through release of urothelial macrophage migration inhibitory factor (MIF) and High Mobility Group Box 1 (HMGB1). We aimed to confirm our observations using transgenic strains and examine downstream mechanisms at the level of the bladder involved in BHA.
Methods: BHA was induced in female mice with intravesical PAR4 (100 µM; 1hr; C57BL/6) or di-sulfide HMGB1 (dsHMGB1, 10 µg; 1hr; MIF knockout). Lower abdominal von Frey (VF) mechanical threshold (index of BHA) was measured before and 24 hr after treatment. Intravesical pre-treatments (10 min prior to PAR4 or dsHMGB1) included: PBS; ethyl pyruvate (EP; inhibiting HMGB1 release); N-acetylcysteine amide (NACA; ROS scavenger); FR180204 (FR; selective ERK1/2 inhibitor; methylcellulose as solvent); LY294002 (LY; PI3k inhibitor; DMSO as solvent). Awake micturition parameters (voided volume; frequency) were assessed 24 hr after treatment. Bladder histology assessed inflammation/edema. R was used for all analyses.
Results: Scrambled peptide did not induce BHA while intravesical PAR4 induced BHA in WT mice (Fig 1A). Pre-treatment with EP nearly blocked BHA (Fig 1B) while NACA significantly reduced it (Fig 1C) and FR had no effect (Fig 1D). PAR4 did not induce BHA in MIF KO mice (Fig 2A) while intravesical dsHMGB1 elicited BHA (Fig 2B). Pre-treatment with NACA (Fig 2B) and FR (Fig 2C) but not LY (Fig 2D) significantly prevented HMGB1-induced bladder pain in MIF KO mice. No significant effects were noted on micturition volume, frequency, inflammation, or edema.
Conclusions: MIF mediates bladder pain through the release of intravesical (likely urothelial) HMGB1. Downstream of HMGB1, ROS production and ERK1/2 activation at the level of the bladder mediate bladder pain. Further dissecting HMGB1 downstream signaling pathway may lead to novel potential therapeutic strategies to treat non-inflammatory bladder pain.
Source of Funding: DK121695 (PLV); AR049610 (RB)
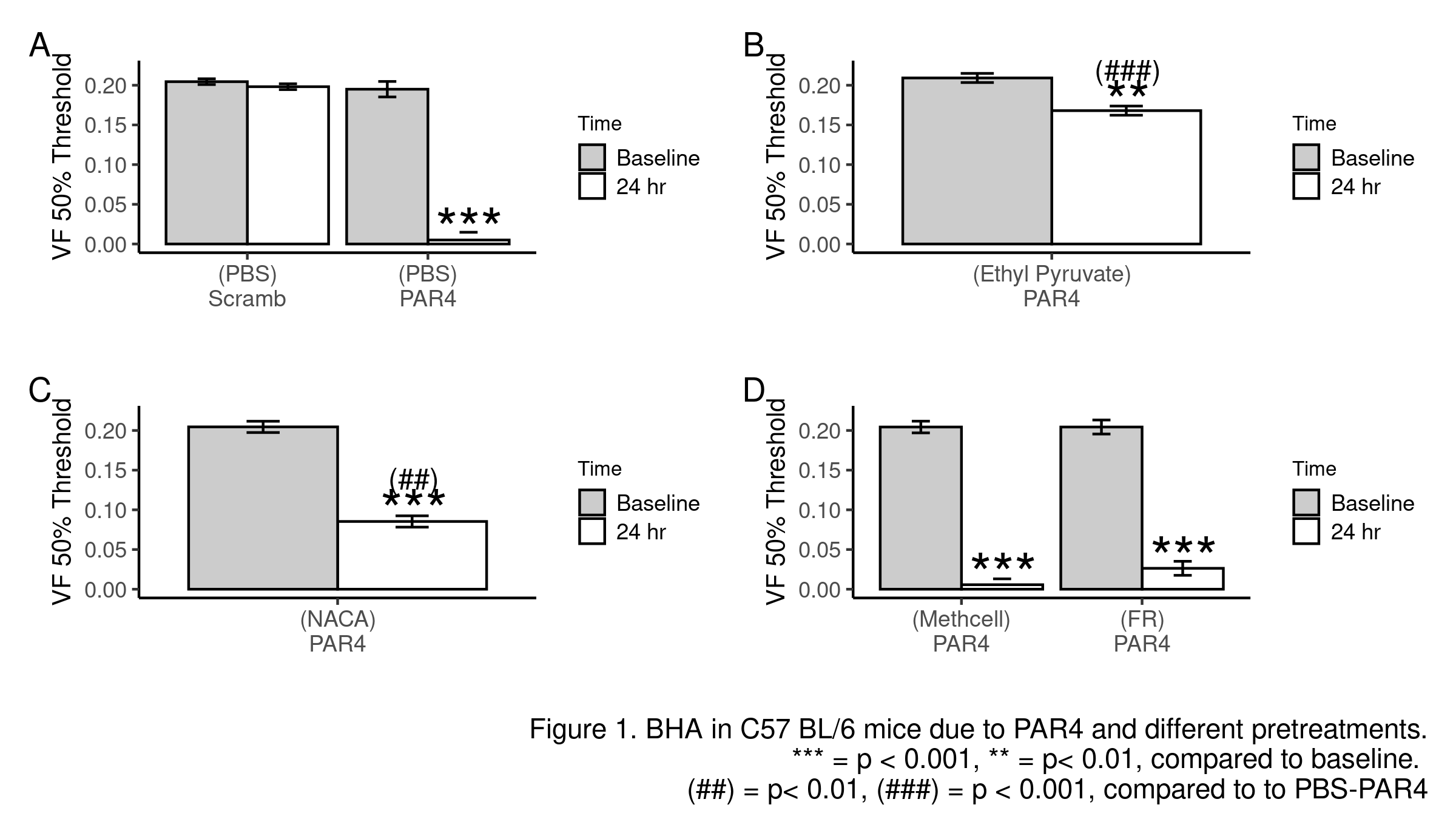
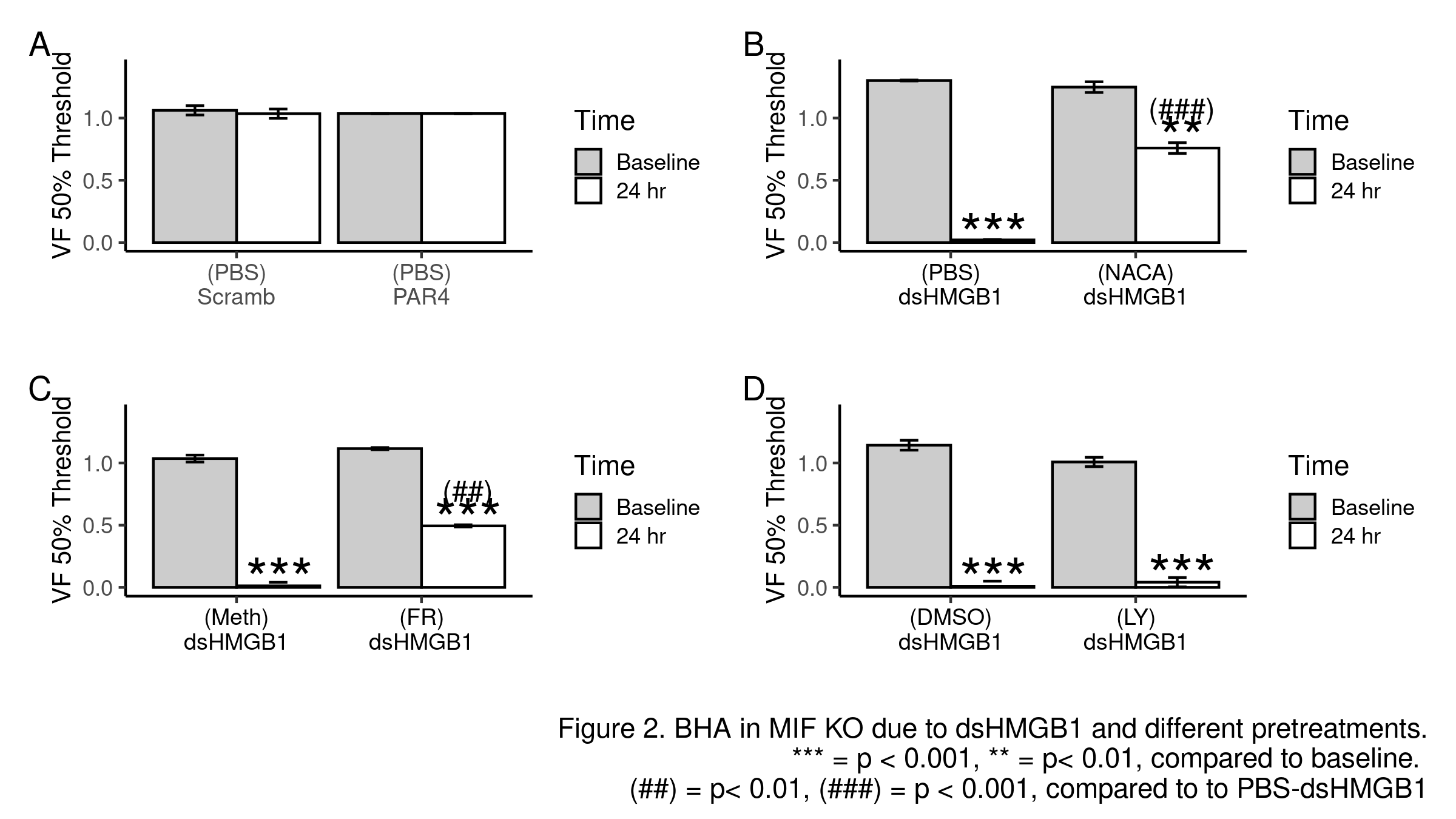
Methods: BHA was induced in female mice with intravesical PAR4 (100 µM; 1hr; C57BL/6) or di-sulfide HMGB1 (dsHMGB1, 10 µg; 1hr; MIF knockout). Lower abdominal von Frey (VF) mechanical threshold (index of BHA) was measured before and 24 hr after treatment. Intravesical pre-treatments (10 min prior to PAR4 or dsHMGB1) included: PBS; ethyl pyruvate (EP; inhibiting HMGB1 release); N-acetylcysteine amide (NACA; ROS scavenger); FR180204 (FR; selective ERK1/2 inhibitor; methylcellulose as solvent); LY294002 (LY; PI3k inhibitor; DMSO as solvent). Awake micturition parameters (voided volume; frequency) were assessed 24 hr after treatment. Bladder histology assessed inflammation/edema. R was used for all analyses.
Results: Scrambled peptide did not induce BHA while intravesical PAR4 induced BHA in WT mice (Fig 1A). Pre-treatment with EP nearly blocked BHA (Fig 1B) while NACA significantly reduced it (Fig 1C) and FR had no effect (Fig 1D). PAR4 did not induce BHA in MIF KO mice (Fig 2A) while intravesical dsHMGB1 elicited BHA (Fig 2B). Pre-treatment with NACA (Fig 2B) and FR (Fig 2C) but not LY (Fig 2D) significantly prevented HMGB1-induced bladder pain in MIF KO mice. No significant effects were noted on micturition volume, frequency, inflammation, or edema.
Conclusions: MIF mediates bladder pain through the release of intravesical (likely urothelial) HMGB1. Downstream of HMGB1, ROS production and ERK1/2 activation at the level of the bladder mediate bladder pain. Further dissecting HMGB1 downstream signaling pathway may lead to novel potential therapeutic strategies to treat non-inflammatory bladder pain.
Source of Funding: DK121695 (PLV); AR049610 (RB)



.jpg)
.jpg)