Back
Poster, Podium & Video Sessions
Best Poster Award
MP07: Bladder & Urethra: Anatomy, Physiology & Pharmacology
MP07-06: Specialized pro-resolution mediators in the bladder: receptor expression and recovery of bladder function from cystitis
Friday, May 13, 2022
10:30 AM – 11:45 AM
Location: Room 228
Francis Hughes*, Armand Allkanjari, Michael Odom, J. Todd Purves, Durham, NC
- FH
Francis Hughes, Jr., PhD
Assistant Professor
Duke University Medical Center
Poster Presenter(s)
Introduction: Inflammation is a causative/exacerbating factor in most benign bladder disorders and control is a balance between initiating and resolving factors. Resolution is mediated by 5 classes of molecules (Specialized Proresolution Mediators-SPMs) functioning through 7 receptors (6 in rodents). We have shown receptor expression in rat bladder and effectiveness of one SPM (Annexin-A1) in alleviating detrimental effects of outlet obstruction. Here we survey receptor expression in the human and mouse bladders. We then examine representatives of 3 additional classes of SPM (Resolvin E1 (RvE1), Maresin 1 (MaR1) and Protectin D1 (PD1)) for in vitro ability to promote wound healing in urothelia (scratch assay) and their in vivo ability to speed the resolution of inflammation induced by cyclophosphamide (CP). Finally, we examined the ability of RvE1 to reverse CP-induced bladder dysfunction (urodynamics) and fibrotic gene expression.
Methods: Receptor expression assessed by immunocytochemistry. Scratch Assay: a scratch (p200 tip) in a urothelial monolayer was photographed at 0 and 24h and the % closure of the wound calculated. For in vivo studies, mice were injected (i.p.) [Day 0-CP (150 mg/kg), Day1-3 SPM (25 µg/kg/day)] then analyzed on day 4. Inflammation was assessed by Evans blue dye extravasation, bladder function by urodynamics and gene expression by qPCR.
Results: All SPM receptors were expressed in human (7) and mice (6). RvE1, MaR1 and PD1 promoted in vitro epithelial wound repair (RvE1 EC90 =12.5 nM). All 3 SPMs reduced CP-induced bladder inflammation while urodynamics revealed that RvE1 reversed CP-induced changes in void volume, frequency and bladder capacity. RvE1 also restored expression of TGF-ß and collagen I to control levels.
Conclusions: The results expand the known SPMs active in the bladder and provide therapeutic targets for controlling inflammation in benign bladder diseases.
Source of Funding: NIH-RO1 DK117890, NIH-K12 DK100024
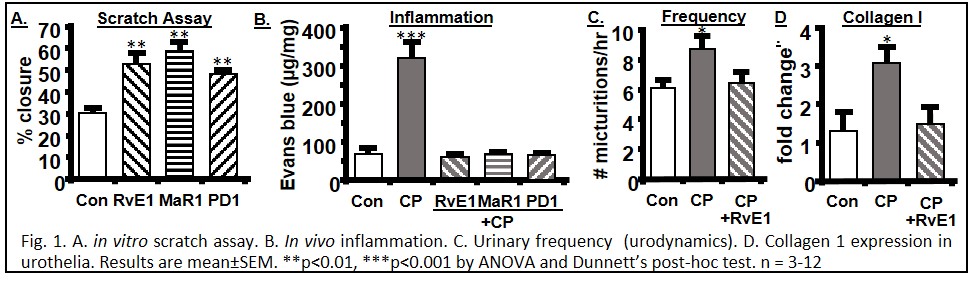
Methods: Receptor expression assessed by immunocytochemistry. Scratch Assay: a scratch (p200 tip) in a urothelial monolayer was photographed at 0 and 24h and the % closure of the wound calculated. For in vivo studies, mice were injected (i.p.) [Day 0-CP (150 mg/kg), Day1-3 SPM (25 µg/kg/day)] then analyzed on day 4. Inflammation was assessed by Evans blue dye extravasation, bladder function by urodynamics and gene expression by qPCR.
Results: All SPM receptors were expressed in human (7) and mice (6). RvE1, MaR1 and PD1 promoted in vitro epithelial wound repair (RvE1 EC90 =12.5 nM). All 3 SPMs reduced CP-induced bladder inflammation while urodynamics revealed that RvE1 reversed CP-induced changes in void volume, frequency and bladder capacity. RvE1 also restored expression of TGF-ß and collagen I to control levels.
Conclusions: The results expand the known SPMs active in the bladder and provide therapeutic targets for controlling inflammation in benign bladder diseases.
Source of Funding: NIH-RO1 DK117890, NIH-K12 DK100024


.jpg)
.jpg)