Back
Poster, Podium & Video Sessions
Moderated Poster
MP07: Bladder & Urethra: Anatomy, Physiology & Pharmacology
MP07-13: Medications mostly associated with haematuria: assessment of the EudraVigilance (EV) and Food and Drug Administration (FDA) Pharmacovigilance databases entries
Friday, May 13, 2022
10:30 AM – 11:45 AM
Location: Room 228
Lorenzo Maria Rovesti*, Rome, Italy, Antonio Nacchia, Ferdinando Di Giacomo, Giuseppe Disabato, Rionero in Vulture, Italy, Carmen Gravina, Giacomo Gallo, Jordi Stira, Beatrice Turchi, Alessandro Guercio, Riccardo Lombardo, Antonio Cicione, Olivia Alessandra Voglino, Valeria Baldassarri, Giorgio Guarnotta, Elisa Mancini, Antonio Franco, Simone D'Annunzio, Andrea Tubaro, Cosimo De Nunzio, Rome, Italy
- LR
Poster Presenter(s)
Introduction: Drugs may have a direct causative role in triggering haematuria. The range of medications which may be responsible for haematuria is wide but little is known on those which are most frequently involved. We aimed at identifying 1) which medications are associated with most haematuria reports; 2) within the high-risk list of medications, comparing their potential to cause haematuria through a disproportionality analysis.
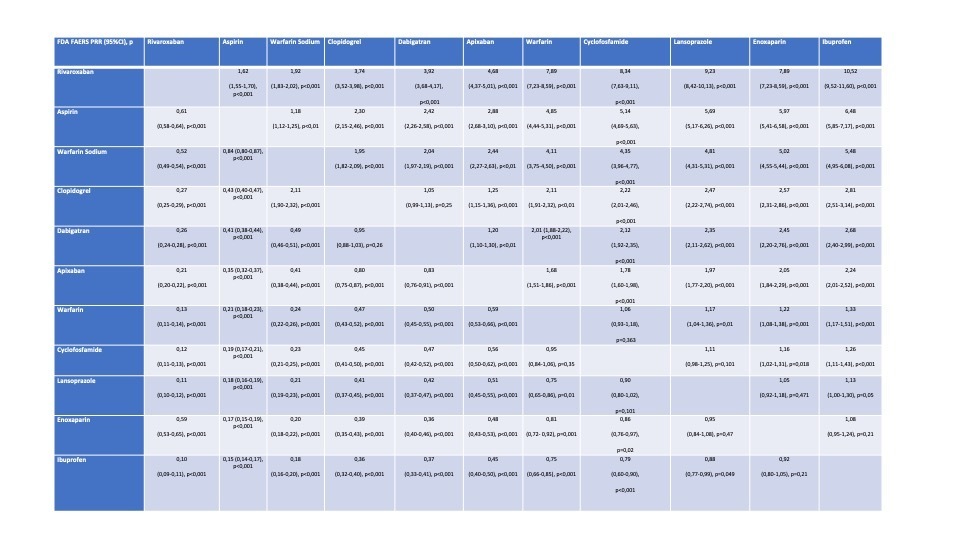
Methods: The Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database was queried to identify the drugs which were associated the most with haematuria individual reports till 30 June 2021 (Table). Only those drugs with a minimum of 400 haematuria reports were here considered for disproportionality analysis. 11 drugs were identified. We recorded the number of haematuria reports for these 11 drugs in EudraVigilance (EV) database. Proportional Reported Ratios (PRRs) were computed for all the drugs individuated in this way..
Results: Overall, 36315 haematuria reports were identified, 15319 of which (43%) were associated with those 11 medications which had a minimum of 400 reports each (Table). Within this group of high-risk medications, Rivaroxaban presented higher risk of haematuria. (Table). Most of the reported haematuria for drugs were presented in the 65-85 range of age (37%). Serious cases including death were 32131 (88%). EV database analysis confirmed the highest rate of haematuria reports for Rivaroxaban (5042 events of which 302 serious (6%), with 31 deaths (1%)) within anticoagulants and for Aspirin (2404 events of which 101 serious (4%) with 18 deaths (1%)) within antiplatelets.
Conclusions: 11 drugs were here identified as being associated with significant reporting levels for haematuria. Among them Rivaroxaban was responsible for the most reports as in FDA FAERS database as in EV database and generated the strongest signal of disproportionate reporting. Prescribers should inform those treated with these medications about the risk of haematuria and its sequelae. These results require to be further integrated with clinical evidence.
Source of Funding: None.
Methods: The Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database was queried to identify the drugs which were associated the most with haematuria individual reports till 30 June 2021 (Table). Only those drugs with a minimum of 400 haematuria reports were here considered for disproportionality analysis. 11 drugs were identified. We recorded the number of haematuria reports for these 11 drugs in EudraVigilance (EV) database. Proportional Reported Ratios (PRRs) were computed for all the drugs individuated in this way..
Results: Overall, 36315 haematuria reports were identified, 15319 of which (43%) were associated with those 11 medications which had a minimum of 400 reports each (Table). Within this group of high-risk medications, Rivaroxaban presented higher risk of haematuria. (Table). Most of the reported haematuria for drugs were presented in the 65-85 range of age (37%). Serious cases including death were 32131 (88%). EV database analysis confirmed the highest rate of haematuria reports for Rivaroxaban (5042 events of which 302 serious (6%), with 31 deaths (1%)) within anticoagulants and for Aspirin (2404 events of which 101 serious (4%) with 18 deaths (1%)) within antiplatelets.
Conclusions: 11 drugs were here identified as being associated with significant reporting levels for haematuria. Among them Rivaroxaban was responsible for the most reports as in FDA FAERS database as in EV database and generated the strongest signal of disproportionate reporting. Prescribers should inform those treated with these medications about the risk of haematuria and its sequelae. These results require to be further integrated with clinical evidence.
Source of Funding: None.


.jpg)
.jpg)