Back
Poster, Podium & Video Sessions
Moderated Poster
MP07: Bladder & Urethra: Anatomy, Physiology & Pharmacology
MP07-20: Centrally administered corticotropin-releasing factor induces facilitation of the rat micturition via brain glutamatergic receptors
Friday, May 13, 2022
10:30 AM – 11:45 AM
Location: Room 228
Takahiro Shimizu*, Yurika Hata, Suo Zou, Masaki Yamamoto, Hideaki Ono, Yohei Shimizu, Takaaki Aratake, Shogo Shimizu, Youichirou Higashi, Takashi Karashima, Motoaki Saito, Nankoku, Japan
- TS
Takahiro Shimizu, PhD
Department of Pharmacology, Kochi Medical School, Kochi University
Poster Presenter(s)
Introduction: Roles of brain corticotropin-releasing factor (CRF), a representative stress-related neuropeptide, in regulation of the micturition are still controversial. In this study, we examined the effects of centrally administered CRF on the rat micturition and central mechanisms for the CRF-induced responses, focusing on brain CRF1/CRF2 receptor subtypes and glutamatergic receptors (NMDA and AMPA subtypes).
Methods: In urethane anesthetized (0.8 g/kg, ip) male Wistar rats (300-450 g), a catheter was inserted into the bladder to perform cystometry (CMG, 12 ml/h saline infusion). CRF (1 or 3 nmol/rat) or vehicle was intracerebroventricularly (icv) administered 3 h after the surgery. In some rats, effects of icv pretreated CP154526 (CP, CRF1 antagonist, 30 or 100 nmol/rat), K41498 (K, CRF2 antagonist, 30 nmol/rat), MK-801 (MK, NMDA receptor antagonist, 3 or 10 nmol/rat) or DNQX (AMPA receptor antagonist, 1 or 3 nmol/rat) on the CRF (3 nmol/rat, icv)-induced responses were examined. Continuous CMG and evaluation of intercontraction intervals (ICI) and maximal voiding pressure (MVP) were started 1 h before the first icv administration. When performing single CMG 3 h after the surgery, after 4-5 times of single CMG, CRF (3 nmol/rat) or vehicle was icv administered, then single CMG was performed during 60-120 min after the administration.
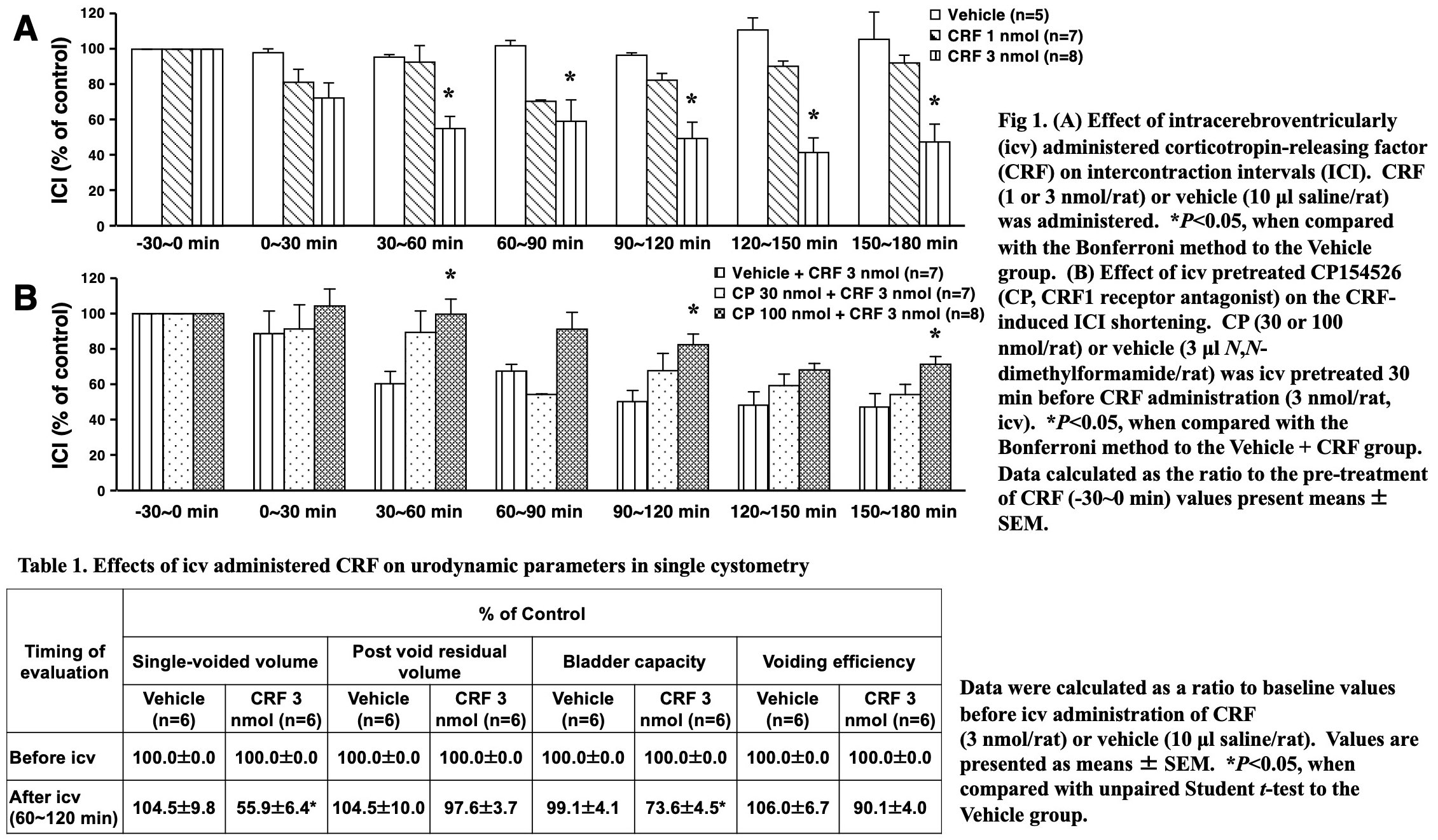
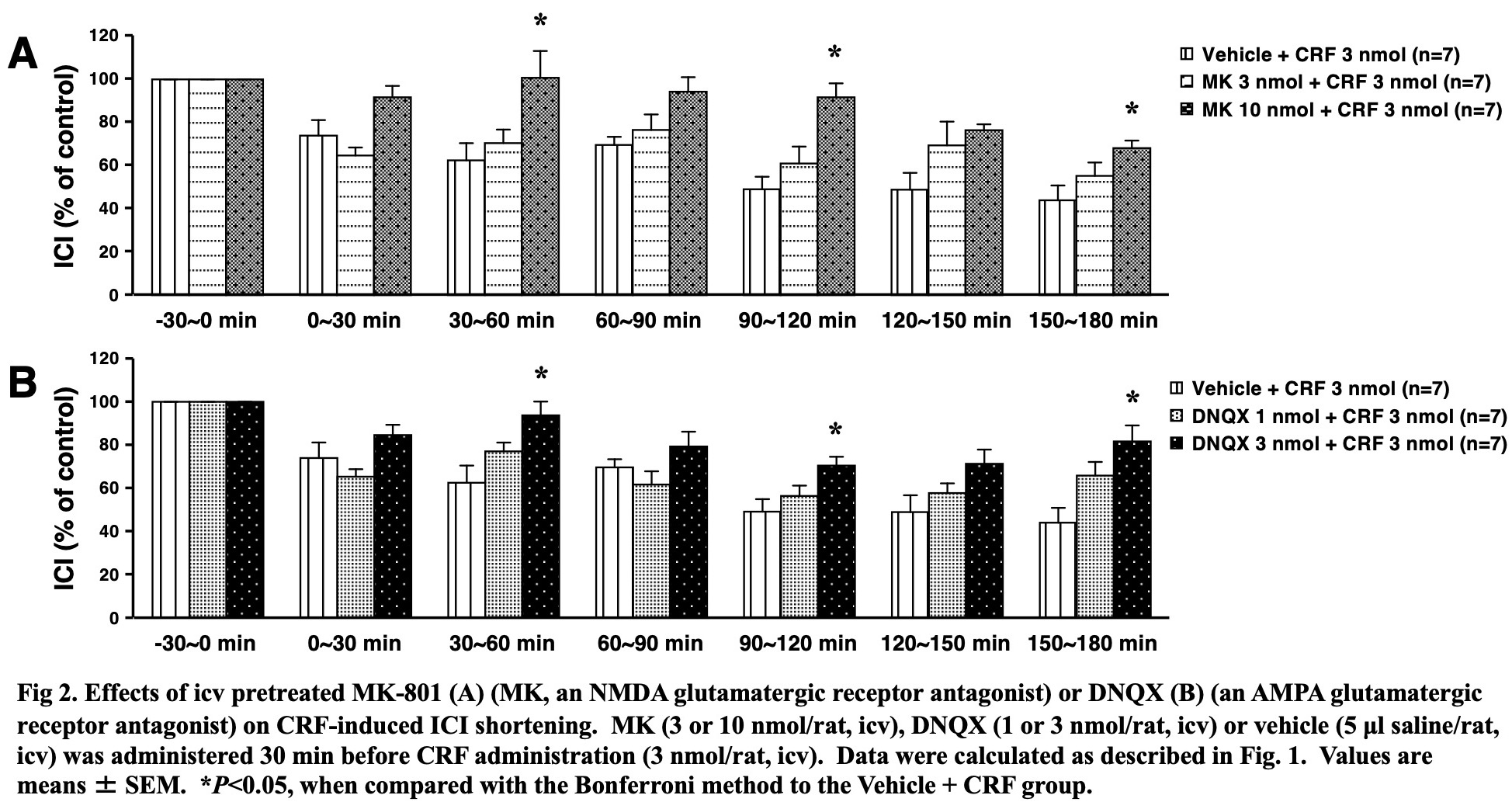
Results: CRF dose-dependently shortened ICI (Fig. 1A) without changing MVP (data not shown). The CRF-induced ICI shortening was suppressed by CP, MK-801 and DNQX (Fig. 1B and Fig. 2), but not by K (data not shown). Compared to the vehicle-treated group, CRF significantly reduced single-voided volume and bladder capacity without changing post void residual volume or voiding efficiency (Table 1).
Conclusions: Centrally administered CRF induces facilitation of the rat micturition via brain CRF1, NMDA and AMPA receptors, therefore, brain CRF1 receptors might be new therapeutic targets for neurogenic bladder overactivity.
Source of Funding: JSPS KAKENHI (#20K07827), Takeda Science Foundation
Methods: In urethane anesthetized (0.8 g/kg, ip) male Wistar rats (300-450 g), a catheter was inserted into the bladder to perform cystometry (CMG, 12 ml/h saline infusion). CRF (1 or 3 nmol/rat) or vehicle was intracerebroventricularly (icv) administered 3 h after the surgery. In some rats, effects of icv pretreated CP154526 (CP, CRF1 antagonist, 30 or 100 nmol/rat), K41498 (K, CRF2 antagonist, 30 nmol/rat), MK-801 (MK, NMDA receptor antagonist, 3 or 10 nmol/rat) or DNQX (AMPA receptor antagonist, 1 or 3 nmol/rat) on the CRF (3 nmol/rat, icv)-induced responses were examined. Continuous CMG and evaluation of intercontraction intervals (ICI) and maximal voiding pressure (MVP) were started 1 h before the first icv administration. When performing single CMG 3 h after the surgery, after 4-5 times of single CMG, CRF (3 nmol/rat) or vehicle was icv administered, then single CMG was performed during 60-120 min after the administration.
Results: CRF dose-dependently shortened ICI (Fig. 1A) without changing MVP (data not shown). The CRF-induced ICI shortening was suppressed by CP, MK-801 and DNQX (Fig. 1B and Fig. 2), but not by K (data not shown). Compared to the vehicle-treated group, CRF significantly reduced single-voided volume and bladder capacity without changing post void residual volume or voiding efficiency (Table 1).
Conclusions: Centrally administered CRF induces facilitation of the rat micturition via brain CRF1, NMDA and AMPA receptors, therefore, brain CRF1 receptors might be new therapeutic targets for neurogenic bladder overactivity.
Source of Funding: JSPS KAKENHI (#20K07827), Takeda Science Foundation



.jpg)
.jpg)