Back
Poster, Podium & Video Sessions
Moderated Poster
MP09: Prostate Cancer: Detection & Screening I
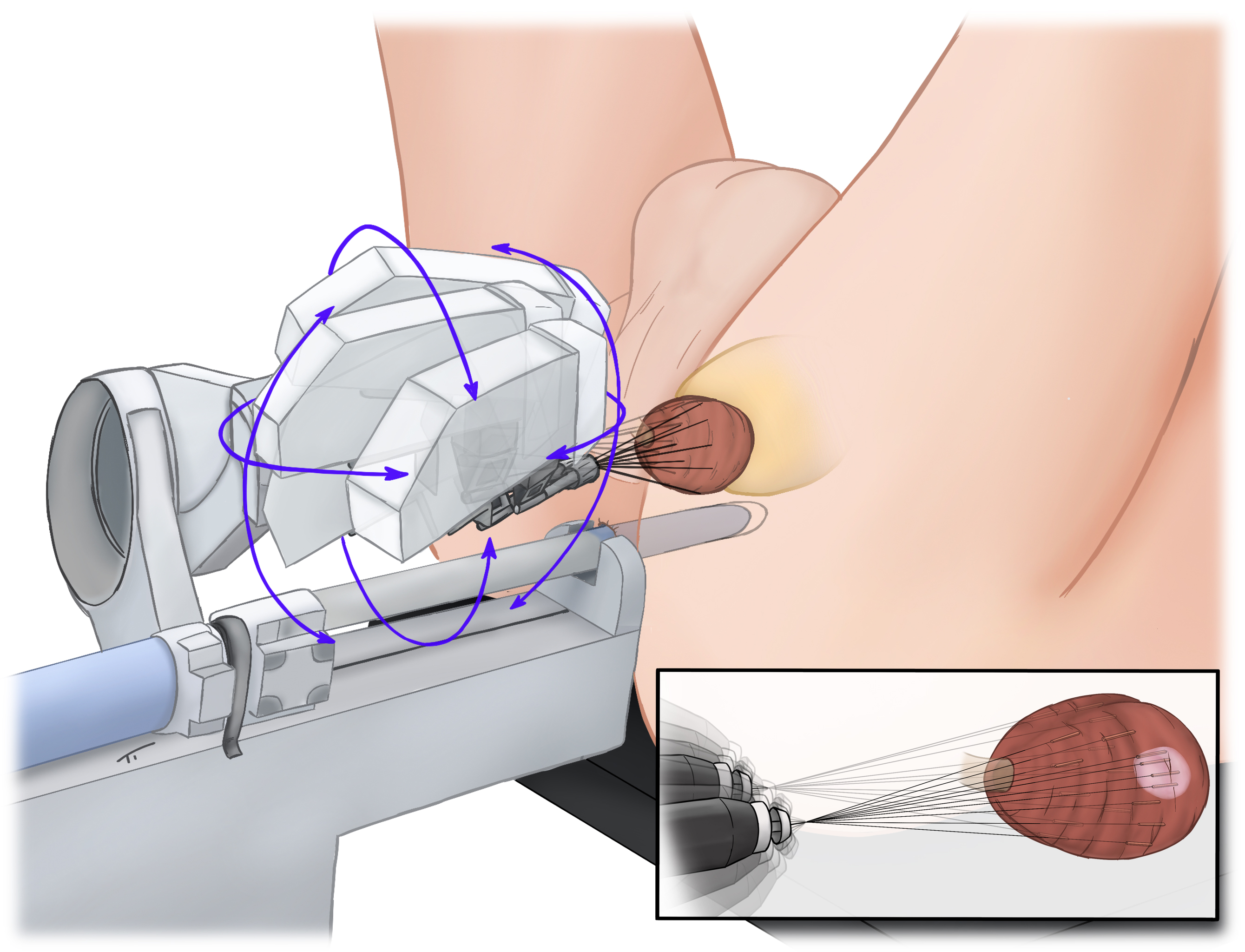
MP09-07: Safety profile of robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate
Friday, May 13, 2022
10:30 AM – 11:45 AM
Location: Room 222
Christian Wetterauer*, Walter Manuel, Pawel Trotsenko, Matthias Marc, Winkel David Jean, Breit Christian, Meyer Anja, Seifert Hans Helge, Basel, Switzerland

Christian Wetterauer
University Hospital Basel
Poster Presenter(s)
Introduction: Robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate (RA-TP-PBx) is a novel and highly accurate procedure. The aim of this study was to evaluate the MonaLisa prostate biopsy system in terms of safety, tolerability, and patient-related outcomes.
Methods: This prospective study included 135 patients, who had undergone RA-TP-PBx at the University Hospital Basel between January 2020 and August 2021. Peri-operative side effects, functional outcomes and patient satisfaction were assessed.
Results: Overall, 18 of 135 patients (13.3%) developed grade I complications according to Clavien-Dindo classification. No higher-grade complications occurred. Mean pain score on the day of biopsy was 1.1 points on VAS, which remained constant on the day after biopsy. Gross haematuria, hematospermia and acute urinary retention occurred in 91/135 (68.9%), 66/135 (26.5%) and 17/135 (12.6%) patients, respectively. One patient (0.7%) developed urinary tract infection.
Conclusions: RA-TP-PBx performed under general anesthesia is a safe and well tolerated procedure. This technique allows to omit perioperative prophylaxis and at the same time minimizes the risk of infectious complications. We attribute the favorable risk profile and tolerability to the minimal invasive approach via two entry points.
Source of Funding: Uromed
Methods: This prospective study included 135 patients, who had undergone RA-TP-PBx at the University Hospital Basel between January 2020 and August 2021. Peri-operative side effects, functional outcomes and patient satisfaction were assessed.
Results: Overall, 18 of 135 patients (13.3%) developed grade I complications according to Clavien-Dindo classification. No higher-grade complications occurred. Mean pain score on the day of biopsy was 1.1 points on VAS, which remained constant on the day after biopsy. Gross haematuria, hematospermia and acute urinary retention occurred in 91/135 (68.9%), 66/135 (26.5%) and 17/135 (12.6%) patients, respectively. One patient (0.7%) developed urinary tract infection.
Conclusions: RA-TP-PBx performed under general anesthesia is a safe and well tolerated procedure. This technique allows to omit perioperative prophylaxis and at the same time minimizes the risk of infectious complications. We attribute the favorable risk profile and tolerability to the minimal invasive approach via two entry points.
Source of Funding: Uromed


.jpg)
.jpg)