Back
Poster, Podium & Video Sessions
Moderated Poster
MP09: Prostate Cancer: Detection & Screening I
MP09-09: The results of transperineal versus transrectal prostate biopsy: an updated systematic review and meta-analysis
Friday, May 13, 2022
10:30 AM – 11:45 AM
Location: Room 222
Jindong Dai*, Jiyu Yang, Chengdu, China, People's Republic of, Xingming Zhang, Chengdu , China, People's Republic of, Pengfei Shen, Hao Zeng, Chengdu, China, People's Republic of
- JD
Poster Presenter(s)
Introduction: This updated systematic review was performed to compare the efficacy and complications of transperineal (TP) vs. transrectal (TR) prostate biopsy.
Methods: Systematic research of PUBMED, EMBASE and the Cochrane Library was performed to identify all clinical controlled trials on prostate cancer (PCa) detection rate and complications achieved by TP and TR biopsies. Prostate biopsies included sextant, extensive and saturation biopsy procedures. All patients were assigned to TR group or TP group. Subgroup analysis was performed according to prostate-specific antigen (PSA) levels, prostate volume, prostate anatomical structure, and digital rectal examination (DRE) findings. The Cochrane Collaboration’s RevMan 5.4 software was used for the meta-analysis.
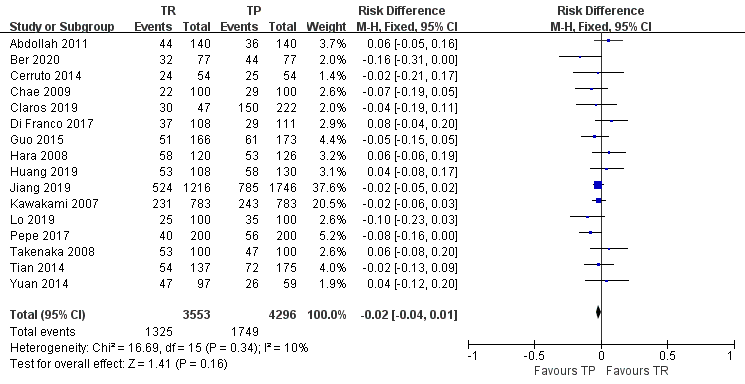
Results: A total of sixteen trials, including eight randomized controlled trials (RCTs) and eight case control studies (CCS), met our inclusion criteria. Meta-analysis for RCTs combined with CCS showed that there was no difference in the cancer detection rate between the extensive TR and TP group (risk difference (RD), -0.01; 95% confidence interval (CI), -0.04–0.02; P=0.47). However, the more prostate cancer was detected in TP group with 10=PSA (RD, 0.03; 95%CI, 0.01-0.05 ;P=0.009).
Conclusions: In the patients with PSA>10ng/ml,TP has more advantages than TR in cancer detection. Moreover, a significantly lower risk of fever and rectal bleeding was reported for TP prostate biopsy.
Source of Funding: 2021YFS0119

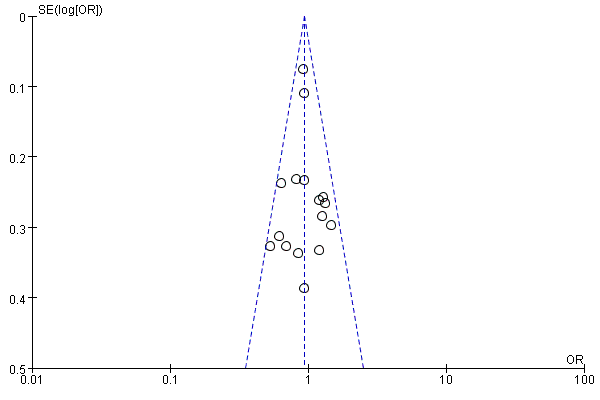

Methods: Systematic research of PUBMED, EMBASE and the Cochrane Library was performed to identify all clinical controlled trials on prostate cancer (PCa) detection rate and complications achieved by TP and TR biopsies. Prostate biopsies included sextant, extensive and saturation biopsy procedures. All patients were assigned to TR group or TP group. Subgroup analysis was performed according to prostate-specific antigen (PSA) levels, prostate volume, prostate anatomical structure, and digital rectal examination (DRE) findings. The Cochrane Collaboration’s RevMan 5.4 software was used for the meta-analysis.
Results: A total of sixteen trials, including eight randomized controlled trials (RCTs) and eight case control studies (CCS), met our inclusion criteria. Meta-analysis for RCTs combined with CCS showed that there was no difference in the cancer detection rate between the extensive TR and TP group (risk difference (RD), -0.01; 95% confidence interval (CI), -0.04–0.02; P=0.47). However, the more prostate cancer was detected in TP group with 10=PSA (RD, 0.03; 95%CI, 0.01-0.05 ;P=0.009).
Conclusions: In the patients with PSA>10ng/ml,TP has more advantages than TR in cancer detection. Moreover, a significantly lower risk of fever and rectal bleeding was reported for TP prostate biopsy.
Source of Funding: 2021YFS0119





.jpg)
.jpg)