Back
Poster, Podium & Video Sessions
Moderated Poster
MP12: Kidney Cancer: Advanced (including Drug Therapy) I
MP12-14: Site-specific early response patterns in advanced RCC patients treated with Nivolumab and Ipilimumab compared with Sunitinib therapy
Friday, May 13, 2022
1:00 PM – 2:15 PM
Location: Room 222
Renpei Kato*, Tomohiko Matsuura, Shigekatsu Maekawa, Yoichiro Kato, Mitsugu Kanehira, Ryo Takata, Wataru Obara, Yahaba, Japan
- RK
Renpei Kato
Iwate Medical University
Poster Presenter(s)
Introduction: In Checkmate 214 trial, Nivolumab and Ipilimumab (Nivo + Ipi) has shown overlapping Kaplan-Meier curves of progression-free survival and overall survival within 6 months compared with Sunitinib arm. This study aimed to identify early response pattern of primary and metastatic sites in advanced renal cell carcinoma (RCC) patients treated with Nivo + Ipi.
Methods: Patients who treated Nivo + Ipi and Sunitinib therapy as first-line therapy were included. To exclude selection bias due to patient characteristics, baseline clinical data was adjusted by inverse probability of treatment weighting (IPTW). CT imaging was obtained at baseline, 12 weeks and 24 weeks from starting first-line therapy. The lesional response rate (LRR) of primary and metastatic sites and the overall response rate (ORR) were determined according to Response Evaluation Criteria in Solid Tumors, version 1.1.
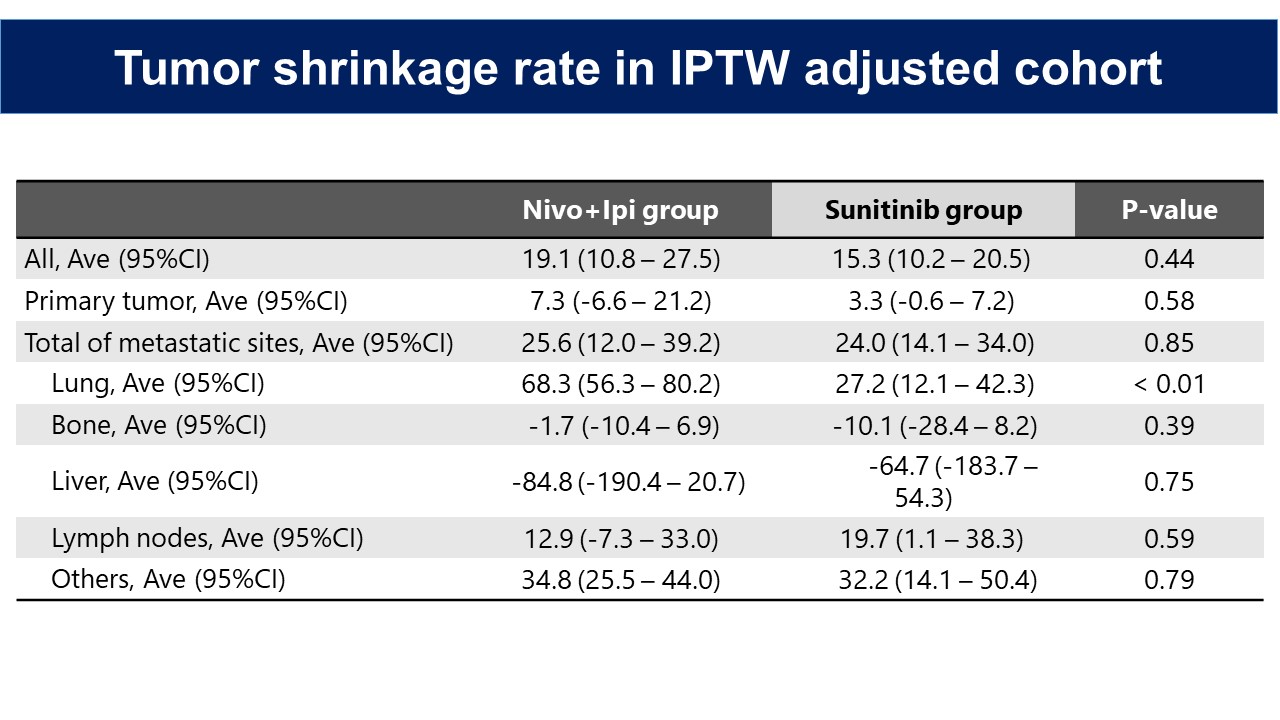
Results: 35 patients were received Nivo + Ipi and 37 were received Sunitinib as the first-line therapy. After IPTW-adjusted analysis, ORR was significantly higher in Nivo +Ipi than in Sunitinib group (49.9% versus 26.8%, p < 0.01). LRR of primary site was significantly higher in Nivo +Ipi than in Sunitinib group (16.9% versus 4.3%, p < 0.01). LRR of metastatic sites were similar between the two groups, but tumor shrinkage rate of lung metastases showed significantly higher in Nivo +Ipi group than in sunitinib group (68.3% versus 27.2%, p < 0.01). Tumor shrinkage rate of metastatic sites correlated significantly with that of primary site in Nivo +Ipi group, but not in Sunitinib group (Nivo + Ipi group: r = 0.69, p < 0.01; Sunitinib group: r = 0.04, p = 0.83).
Conclusions: Our study found that lung metastases exhibited early response to Nivo + Ipi therapy. Further studies are warranted to verify whether site-specific early response predicts the prolongation of survival benefit of advanced RCC patients treated with Nivo + Ipi therapy in the clinical setting.
Source of Funding: All authors declare no potential conflicts of interest.
Methods: Patients who treated Nivo + Ipi and Sunitinib therapy as first-line therapy were included. To exclude selection bias due to patient characteristics, baseline clinical data was adjusted by inverse probability of treatment weighting (IPTW). CT imaging was obtained at baseline, 12 weeks and 24 weeks from starting first-line therapy. The lesional response rate (LRR) of primary and metastatic sites and the overall response rate (ORR) were determined according to Response Evaluation Criteria in Solid Tumors, version 1.1.
Results: 35 patients were received Nivo + Ipi and 37 were received Sunitinib as the first-line therapy. After IPTW-adjusted analysis, ORR was significantly higher in Nivo +Ipi than in Sunitinib group (49.9% versus 26.8%, p < 0.01). LRR of primary site was significantly higher in Nivo +Ipi than in Sunitinib group (16.9% versus 4.3%, p < 0.01). LRR of metastatic sites were similar between the two groups, but tumor shrinkage rate of lung metastases showed significantly higher in Nivo +Ipi group than in sunitinib group (68.3% versus 27.2%, p < 0.01). Tumor shrinkage rate of metastatic sites correlated significantly with that of primary site in Nivo +Ipi group, but not in Sunitinib group (Nivo + Ipi group: r = 0.69, p < 0.01; Sunitinib group: r = 0.04, p = 0.83).
Conclusions: Our study found that lung metastases exhibited early response to Nivo + Ipi therapy. Further studies are warranted to verify whether site-specific early response predicts the prolongation of survival benefit of advanced RCC patients treated with Nivo + Ipi therapy in the clinical setting.
Source of Funding: All authors declare no potential conflicts of interest.


.jpg)
.jpg)