Back
Poster, Podium & Video Sessions
Moderated Poster
MP12: Kidney Cancer: Advanced (including Drug Therapy) I
MP12-15: Effects of first-line immune checkpoint inhibitors in patients with metastatic renal cell carcinoma not meeting trial eligibility criteria
Friday, May 13, 2022
1:00 PM – 2:15 PM
Location: Room 222
Yuki Nemoto*, Saitama, Japan, Hiroki Ishihara, Tokyo, Japan, Kazutaka Nakamura, Fukushima, Japan, Hidekazu Tachibana, Saitama, Japan, Hironori Fukuda, Kazuhiko Yoshida, Hirohito Kobayashi, Junpei Iizuka, Tokyo, Japan, Hiroaki Shimmura, Fukushima, Japan, Yasunobu Hashimoto, Saitama, Japan, Kazunari Tanabe, Tsunenori Kondo, Toshio Takagi, Tokyo, Japan
- YN
Poster Presenter(s)
Introduction: The efficacy of systemic therapy with immune checkpoint inhibitors (ICIs) for metastatic renal cell carcinoma (mRCC) has been demonstrated based on results of clinical trials; however, in trial-ineligible patients who do not meet the eligibility criteria for trials, its efficacy remains unclear.
Methods: We retrospectively evaluated 93 mRCC patients treated with ICIs including nivolumab plus ipilimumab, pembrolizumab plus axitinib, or avelumab plus axitinib as first-line therapy. The criteria for ineligibility for clinical trials was having at least one of the following: Karnofsky performance status (KPS) <70%, hemoglobin level <9.0 g/dL, eGFR <40 mL/min/1.73 m2, platelet count <100000 /µL, neutrophil count <1500 /µL, nonclear-cell histology, and brain metastasis. The patients were divided into two groups based on the ineligibility criteria, and progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were compared between the groups.
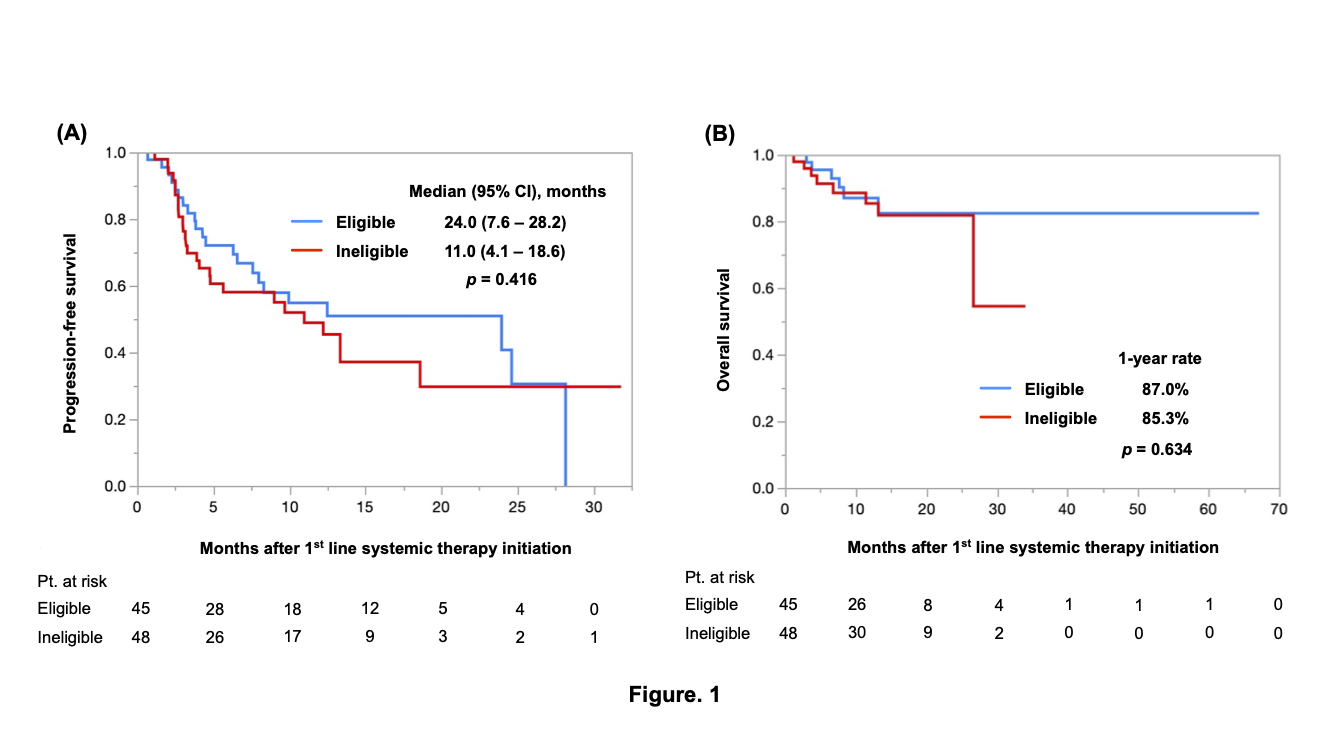
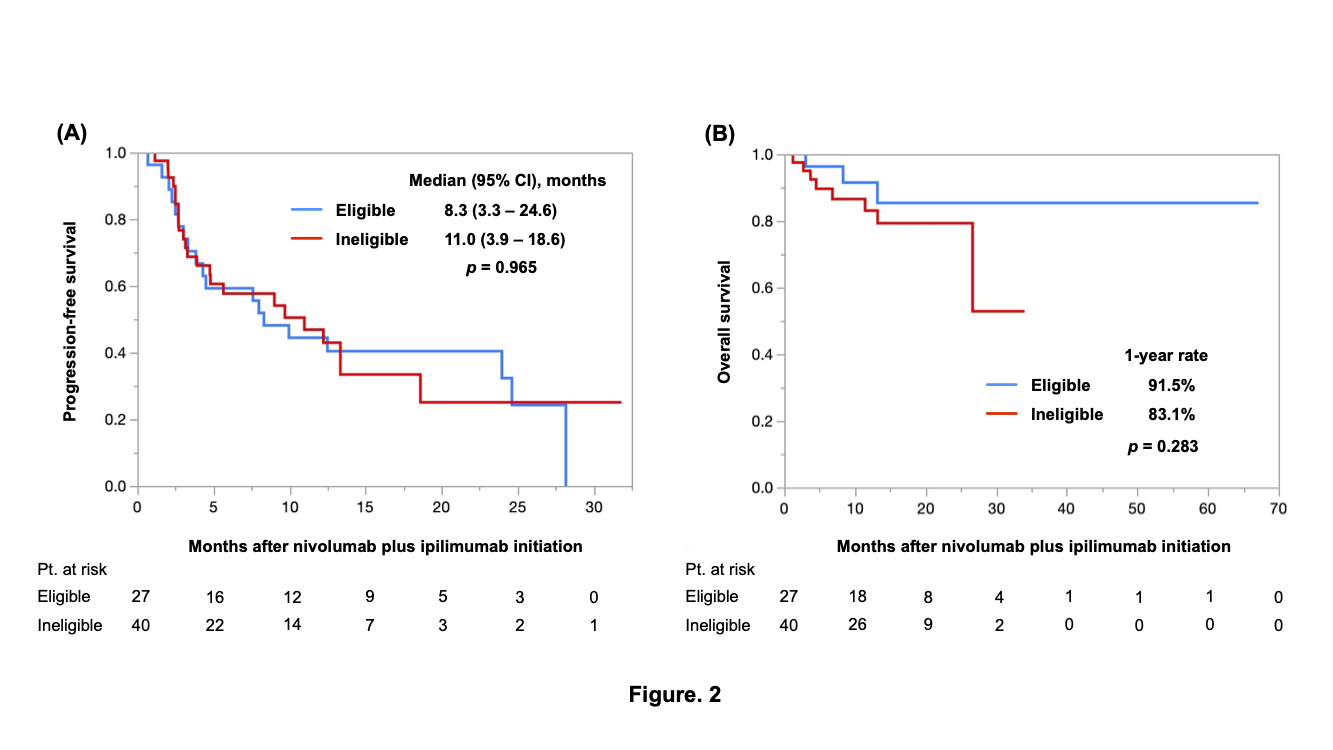
Results: Of the 93 patients, 48 (52%) were classified into the trial-ineligible group. The most frequent reason for being ineligible was a low eGFR (n=20, 45%), followed by nonclear cell histology (n=17, 36%) and low KPS (n=12, 25%). There was no significant difference in the PFS (median PFS: 24.0 vs. 11.0 months, p=0.416), OS (1-year OS: 87.0% vs.85.3%, p=0.634), or ORR (52% vs. 42%, p=0.308) between the trial-eligible and trial-ineligible groups (Figure 1A and 1B). In patients treated with nivolumab plus ipilimumab (n=67), the PFS (median PFS: 8.3 vs. 11.0 months, p=0.965), OS (1-year OS: 91.5% vs.83.1%, p=0.283), and ORR (50% vs. 45%, p=0.691) did not differ between the two groups (Figure 2A and 2B).
Conclusions: In a real-world setting, a substantial number of patients did not meet the eligibility criteria for clinical trials. Even in such patients, ICI-based treatment exhibited feasible anti-tumor activity, compared to that in trial-eligible patients.
Source of Funding: None
Methods: We retrospectively evaluated 93 mRCC patients treated with ICIs including nivolumab plus ipilimumab, pembrolizumab plus axitinib, or avelumab plus axitinib as first-line therapy. The criteria for ineligibility for clinical trials was having at least one of the following: Karnofsky performance status (KPS) <70%, hemoglobin level <9.0 g/dL, eGFR <40 mL/min/1.73 m2, platelet count <100000 /µL, neutrophil count <1500 /µL, nonclear-cell histology, and brain metastasis. The patients were divided into two groups based on the ineligibility criteria, and progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) were compared between the groups.
Results: Of the 93 patients, 48 (52%) were classified into the trial-ineligible group. The most frequent reason for being ineligible was a low eGFR (n=20, 45%), followed by nonclear cell histology (n=17, 36%) and low KPS (n=12, 25%). There was no significant difference in the PFS (median PFS: 24.0 vs. 11.0 months, p=0.416), OS (1-year OS: 87.0% vs.85.3%, p=0.634), or ORR (52% vs. 42%, p=0.308) between the trial-eligible and trial-ineligible groups (Figure 1A and 1B). In patients treated with nivolumab plus ipilimumab (n=67), the PFS (median PFS: 8.3 vs. 11.0 months, p=0.965), OS (1-year OS: 91.5% vs.83.1%, p=0.283), and ORR (50% vs. 45%, p=0.691) did not differ between the two groups (Figure 2A and 2B).
Conclusions: In a real-world setting, a substantial number of patients did not meet the eligibility criteria for clinical trials. Even in such patients, ICI-based treatment exhibited feasible anti-tumor activity, compared to that in trial-eligible patients.
Source of Funding: None



.jpg)
.jpg)