Back
Poster, Podium & Video Sessions
Moderated Poster
MP12: Kidney Cancer: Advanced (including Drug Therapy) I
MP12-18: Immune-related adverse events associated with better survival outcomes for metastatic renal cell carcinoma treated with nivolumab plus ipilimumab
Friday, May 13, 2022
1:00 PM – 2:15 PM
Location: Room 222
Kazuyuki Numakura*, Akita, Japan, Shingo Hatakeyama, Hirosaki, Japan, Yuya Sekine, Yumina Muto, Mizuki Kobayashi, Soki Kashima, Ryohei Yamamoto, Atsushi Koizumi, Taketoshi Nara, Mitsuru Saito, Shintaro Narita, Akita, Japan, Chikara Ohyama, Hirosaki, Japan, Tomonori Habuchi, Akita, Japan

Kazuyuki Numakura, MD
Akita University Graduate School of Medicine
Poster Presenter(s)
Introduction: A combination immune therapy by nivolumab and ipilimumab (NIVO+IPI) is the first-line treatment for metastatic renal cell carcinoma (mRCC) patients with IMDC intermediate and poor risk. Almost 40 % of patients achieved a durable response, and median overall survival (OS) expects nearly four years. On the other hand, since 20% of patients conceive a primary resistant disease to NIVO+IPI, a predictive marker for clinical outcomes of the NIVO+IPI must be demanded. The impact of immune-related adverse events (irAEs) occurring from NIVO+IPI is not well investigated yet. We aimed to evaluate the clinical implication of irAEs in mRCC patients to select a better candidate to be administrated NIVO+IPI.
Methods: We retrospectively evaluated 89 patients with mRCC treated with NIVO+IPI from multiple institutes. The associations between irAE and progression-free survival (PFS), overall survival (OS), and objective response rates (ORRs) were analyzed. Other clinical factors also evaluated the relationship with outcomes.
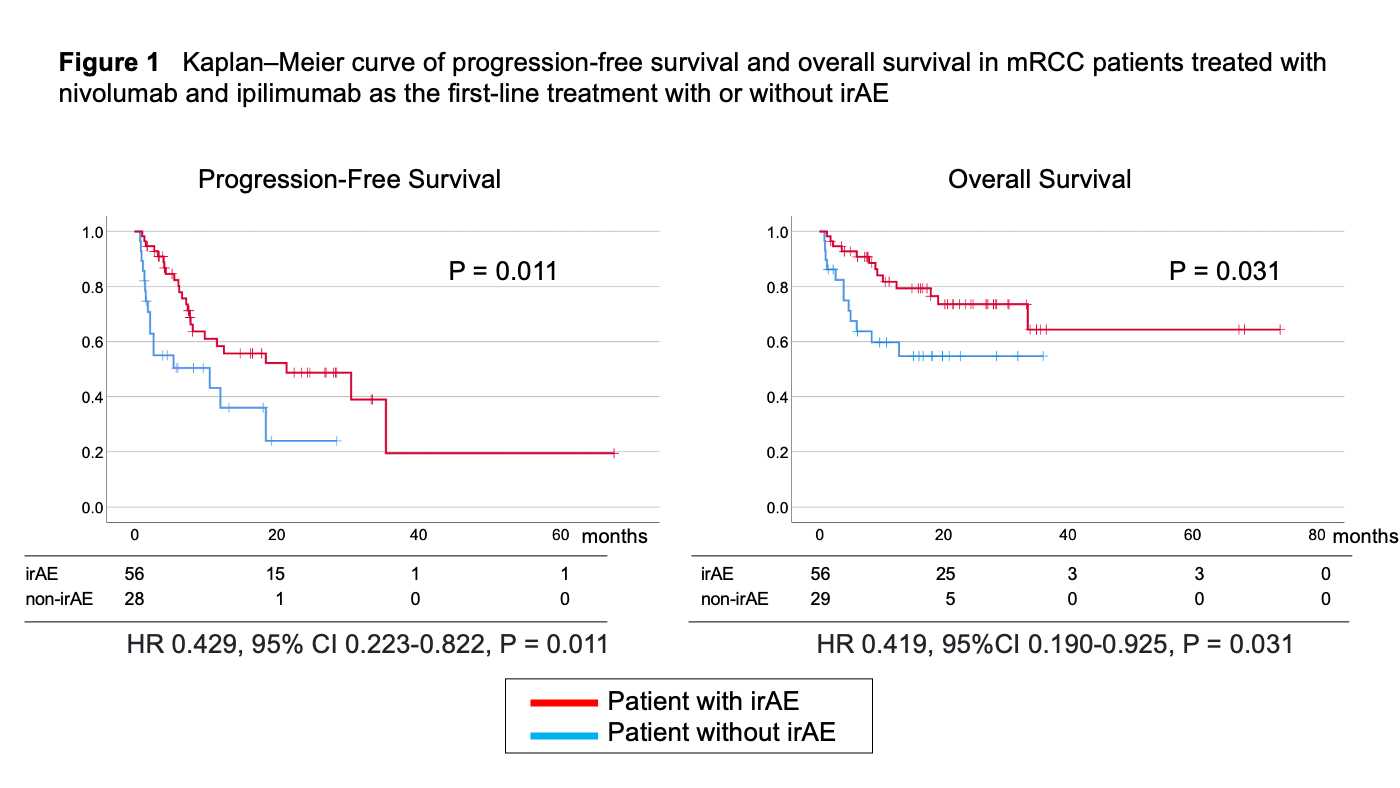
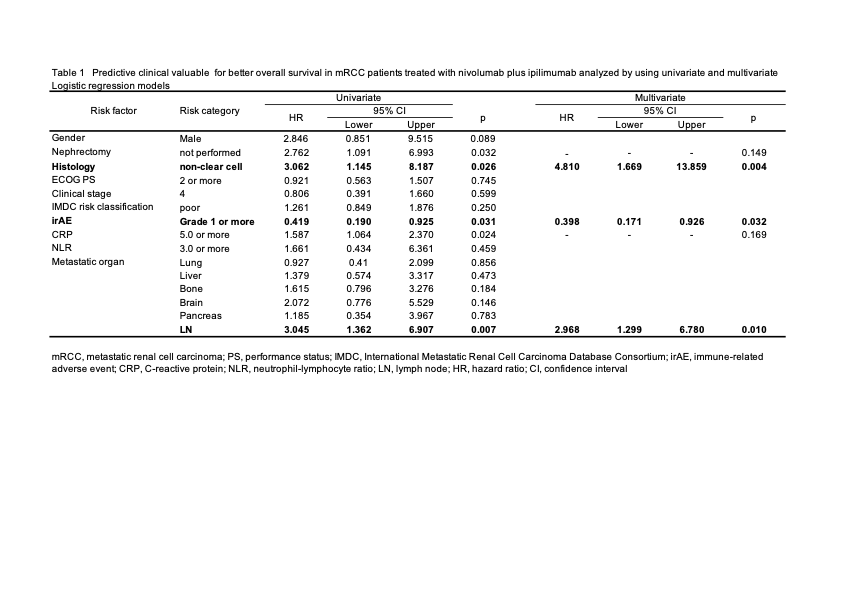
Results: The median observation period was 15.1 months (1 to 74 months). A total of 82 irAEs occurred in 56 patients (63%). PFS and OS were significantly longer in patients with irAEs than that in patients without irAEs (HR 0.429, 95% CI 0.223-0.822, p = 0.011 and HR 0.419, 95%CI 0.190-0.925,P = 0.031) (Figure 1). The univariate analysis resulted that metastasis in lymph node, high CRP, prior nephrectomy, non irAE, and variant histology were associated with worse OS (Table 1). In multivariable analysis, the development of irAEs was an independent predictor of a longer OS (HR 0.398, 95%CI 0.171-0.926, p = 0.032) even analyzed with other clinical significant factors (Table 1). The ORRs were also better in patients with irAEs (OR 3.938, 95%CI 1.378-11.185, P = 0.010).
Conclusions: This retrospective study showed that irAE was associated with PFS and OS independently in patients treated with NIVO+IPI as first-line therapy. The development of irAEs may expect better survival outcomes.
Source of Funding: None
Methods: We retrospectively evaluated 89 patients with mRCC treated with NIVO+IPI from multiple institutes. The associations between irAE and progression-free survival (PFS), overall survival (OS), and objective response rates (ORRs) were analyzed. Other clinical factors also evaluated the relationship with outcomes.
Results: The median observation period was 15.1 months (1 to 74 months). A total of 82 irAEs occurred in 56 patients (63%). PFS and OS were significantly longer in patients with irAEs than that in patients without irAEs (HR 0.429, 95% CI 0.223-0.822, p = 0.011 and HR 0.419, 95%CI 0.190-0.925,P = 0.031) (Figure 1). The univariate analysis resulted that metastasis in lymph node, high CRP, prior nephrectomy, non irAE, and variant histology were associated with worse OS (Table 1). In multivariable analysis, the development of irAEs was an independent predictor of a longer OS (HR 0.398, 95%CI 0.171-0.926, p = 0.032) even analyzed with other clinical significant factors (Table 1). The ORRs were also better in patients with irAEs (OR 3.938, 95%CI 1.378-11.185, P = 0.010).
Conclusions: This retrospective study showed that irAE was associated with PFS and OS independently in patients treated with NIVO+IPI as first-line therapy. The development of irAEs may expect better survival outcomes.
Source of Funding: None



.jpg)
.jpg)