Back
Poster, Podium & Video Sessions
Moderated Poster
MP12: Kidney Cancer: Advanced (including Drug Therapy) I
MP12-20: First-Line Immunotherapy-Based Combinations for Metastatic Renal Cell Carcinoma: Systematic Review and Network Meta-Analysis
Friday, May 13, 2022
1:00 PM – 2:15 PM
Location: Room 222
Fahad Quhal*, Keiichiro Mori, Ekaterina Laukhtina, Benjamin Pradere, Hadi Mostafaei, Shahrokh F. Shariat, Manuela Schmidinger, Vienna, Austria

Fahad Quhal, MD
Medical University of Vienna
Poster Presenter(s)
Introduction: There have been substantial changes in the management of patients with metastatic renal cell carcinoma (mRCC) over the past decade, with upfront immunotherapy-based combinations replacing targeted therapies. A broad range of combinations have been approved, and comparisons of their efficacy is needed to guide the optimal choice of first-line therapy.
Methods: We searched multiple databases and abstracts of major scientific meetings up to August 2021 to identify phase III randomized controlled trials of patients receiving first-line ICI-based combination therapies for mRCC. Progression-free survival (PFS) and overall survival (OS) were the primary endpoints. The secondary endpoints included complete response rates (CRR) and objective response rates (ORR). Subgroup network meta-analyses were performed based on patients’ risk group categories and programmed death ligand-1 (PD-L1) expression status.
Results: Six trials were included in our network meta-analyses comprising 5,121 patients. Nivolumab plus cabozantinib had the highest likelihood of providing the maximal OS (P score: 0. 7573). Lenvatinib plus pembrolizumab demonstrated the highest likelihood of PFS (P score: 0. 9906) and ORR (P score: 0. 9564). CRRs were more likely to be associated with nivolumab plus ipilimumab (P score: 0. 8682). In patients with =1% PD-L1 expression, the highest likelihood of better PFS was associated with lenvatinib plus pembrolizumab and nivolumab plus ipilimumab
Conclusions: Our network meta-analysis suggests that ICI-TKI combinations provide superior PFS, ORR, and OS over ICI-ICI combinations, regardless of the IMDC risk group. However, ICI-ICI combination could be the optimal treatment for tumors with increased PD-L1 expression.
Source of Funding: No
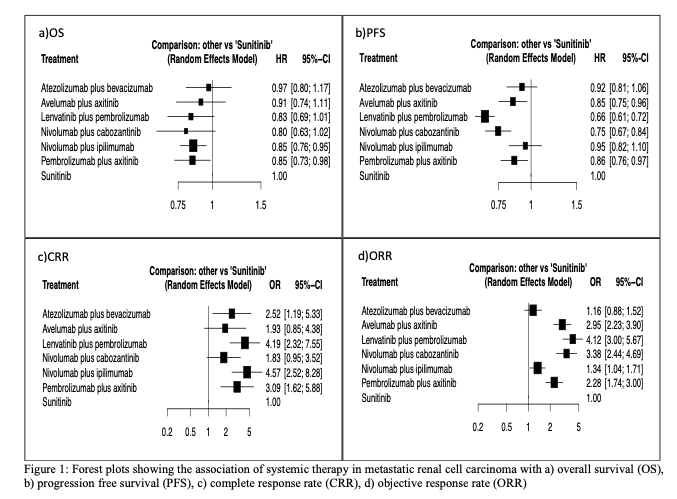


Methods: We searched multiple databases and abstracts of major scientific meetings up to August 2021 to identify phase III randomized controlled trials of patients receiving first-line ICI-based combination therapies for mRCC. Progression-free survival (PFS) and overall survival (OS) were the primary endpoints. The secondary endpoints included complete response rates (CRR) and objective response rates (ORR). Subgroup network meta-analyses were performed based on patients’ risk group categories and programmed death ligand-1 (PD-L1) expression status.
Results: Six trials were included in our network meta-analyses comprising 5,121 patients. Nivolumab plus cabozantinib had the highest likelihood of providing the maximal OS (P score: 0. 7573). Lenvatinib plus pembrolizumab demonstrated the highest likelihood of PFS (P score: 0. 9906) and ORR (P score: 0. 9564). CRRs were more likely to be associated with nivolumab plus ipilimumab (P score: 0. 8682). In patients with =1% PD-L1 expression, the highest likelihood of better PFS was associated with lenvatinib plus pembrolizumab and nivolumab plus ipilimumab
Conclusions: Our network meta-analysis suggests that ICI-TKI combinations provide superior PFS, ORR, and OS over ICI-ICI combinations, regardless of the IMDC risk group. However, ICI-ICI combination could be the optimal treatment for tumors with increased PD-L1 expression.
Source of Funding: No




.jpg)
.jpg)