Back
Poster, Podium & Video Sessions
Moderated Poster
MP13: Health Services Research: Practice Patterns, Quality of Life and Shared Decision Making I
MP13-04: OnabotulinumtoxinA Improves Idiopathic Overactive Bladder Symptoms in Patients Refractory to Oral Medications
Friday, May 13, 2022
2:45 PM – 4:00 PM
Location: Room 228
Elisabeth Carlsson Farrelly*, Stockholm, Sweden, Maria-Fernando Lorenzo-Gomez, Salamanca, Spain, Heinrich Schulte-Baukloh, Berlin, Germany, Mariana Nelson, Irvine, CA, Rizwan Hamid, London, United Kingdom
- EF
Elisabeth Carlsson Farrelly, MD,PhD,FEBU
Consultant urologist
Karolinska Institute, Stockholm, Sweden
Poster Presenter(s)
Introduction: There is a paucity of data comparing the efficacy of onabotulinumtoxinA (onabotA) treatment between patients treated with one oral overactive bladder (OAB) medication to those treated with more than one. This real-world study examines UI (urinary incontinence) episodes and treatment benefit of onabotA in patients who are refractory to one or more oral medications.
Methods: A prospective, observational study (NCT02161159) enrolled adult patients with OAB symptoms inadequately managed by oral medications. Patients were naïve to botulinum toxin for OAB; efficacy and safety analyses were conducted on those that received >1 dose of onabotA. Adverse events (AEs) and adverse drug reactions (ADRs) were recorded for up to 12 months after onabotA treatment. We analyzed UI episodes at baseline for all patients taking oral medications (ß-3 adrenergic agonist [ß-3] and/or an anticholinergic [AC]) for OAB. Only patients taking oral medications before, but not after onabotA and who had >1 diary entry at the indicated timepoint were included in analyses of UI episodes after onabotA at 1 and 12 weeks and treatment benefit scores (TBS) at 12 weeks.
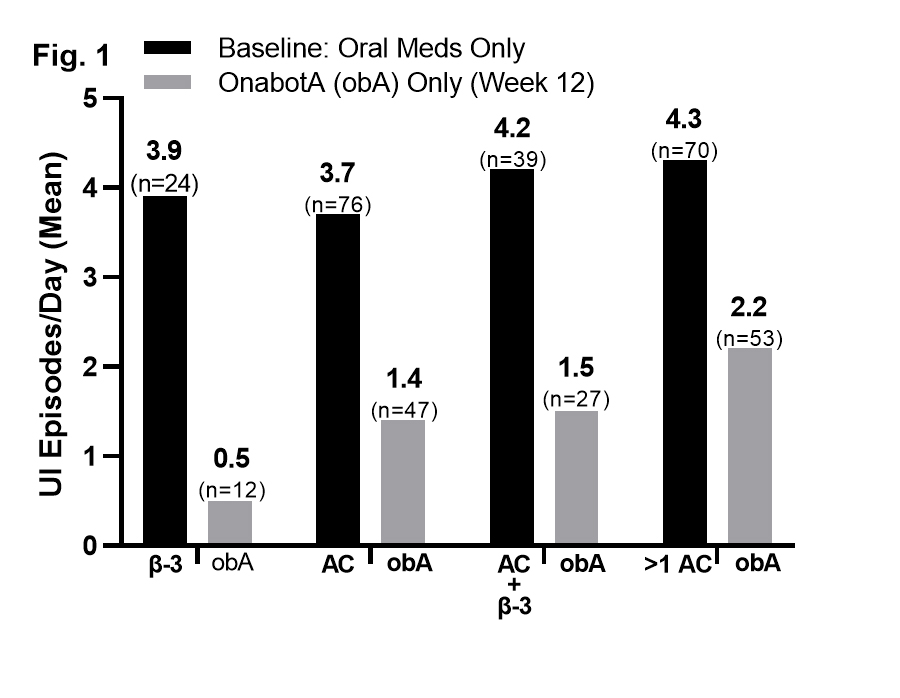
Results: Baseline UI episodes were similar in patients treated with one versus more than one oral medication; reductions in UI at week 12 post-onabotA did not differ based on the number of prior oral medications (Fig. 1). UI was significantly reduced (*P= <.001) in as little as 1 week after onabotA for all prior oral treatment groups (ß-3, -3.3*, n=16; AC, -1.8*, n=53; ß-3 + AC, -2.2*, n=28; >1 AC, - 1.7*, n=52). Of the 233 patients who reported TBS at week 12, 88% were improved or greatly improved after onabotA. In the safety population (N=504), 57 AEs were reported in 38 patients (7.5%); 9 were serious. Urinary retention, as determined by the treating physician, was reported in 5 patients (1.0%); 1 was severe. Symptomatic urinary tract infection was reported in 2 patients (0.4%).
Conclusions: Treatment with onabotA led to significant reductions in UI episodes, with no significant improvement in patients who had been on more than one oral as compared to only one oral before onabotA treatment. Given these results, clinicians may want to consider onabotA treatment earlier as opposed to cycling through oral medications.
Source of Funding: AbbVie
Methods: A prospective, observational study (NCT02161159) enrolled adult patients with OAB symptoms inadequately managed by oral medications. Patients were naïve to botulinum toxin for OAB; efficacy and safety analyses were conducted on those that received >1 dose of onabotA. Adverse events (AEs) and adverse drug reactions (ADRs) were recorded for up to 12 months after onabotA treatment. We analyzed UI episodes at baseline for all patients taking oral medications (ß-3 adrenergic agonist [ß-3] and/or an anticholinergic [AC]) for OAB. Only patients taking oral medications before, but not after onabotA and who had >1 diary entry at the indicated timepoint were included in analyses of UI episodes after onabotA at 1 and 12 weeks and treatment benefit scores (TBS) at 12 weeks.
Results: Baseline UI episodes were similar in patients treated with one versus more than one oral medication; reductions in UI at week 12 post-onabotA did not differ based on the number of prior oral medications (Fig. 1). UI was significantly reduced (*P= <.001) in as little as 1 week after onabotA for all prior oral treatment groups (ß-3, -3.3*, n=16; AC, -1.8*, n=53; ß-3 + AC, -2.2*, n=28; >1 AC, - 1.7*, n=52). Of the 233 patients who reported TBS at week 12, 88% were improved or greatly improved after onabotA. In the safety population (N=504), 57 AEs were reported in 38 patients (7.5%); 9 were serious. Urinary retention, as determined by the treating physician, was reported in 5 patients (1.0%); 1 was severe. Symptomatic urinary tract infection was reported in 2 patients (0.4%).
Conclusions: Treatment with onabotA led to significant reductions in UI episodes, with no significant improvement in patients who had been on more than one oral as compared to only one oral before onabotA treatment. Given these results, clinicians may want to consider onabotA treatment earlier as opposed to cycling through oral medications.
Source of Funding: AbbVie


.jpg)
.jpg)