Back
Poster, Podium & Video Sessions
Moderated Poster
MP18: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Non-neurogenic Voiding Dysfunction I
MP18-06: Efficacy of pulsed electromagnetic field (PEMF) therapy for pelvic pain reduction and symptom improvement in interstitial cystitis: A pilot study
Friday, May 13, 2022
4:30 PM – 5:45 PM
Location: Room 222
Dylan T. Wolff*, Peyton Lee, Christina Ross, Robert J. Evans, Gopal Badlani, Tyler Overholt, Raymond Xu, Catherine A. Matthews, Stephen J. Walker, Winston-Salem, NC
- DW
Poster Presenter(s)
Introduction: PEMF therapy is effective for pain reduction in select chronic pain disorders. The present study evaluated the efficacy of PEMF therapy for pain/symptom management in women with non-bladder centric interstitial cystitis/bladder pain syndrome (IC/BPS).
Methods: Women (ages 18-80 yo) with a confirmed diagnosis of IC/BPS, a numeric rating scale (NRS) score for pelvic pain = 6, and an anesthetic bladder capacity (BC) >400ml underwent 8-minute full body PEMF sessions, twice daily, for 4 weeks. The primary outcome measure was a reduction in NRS score = 2-points. The O’Leary Sant Interstitial Cystitis Symptom and Problem Indices (ICSI/ICPI), Pain and Urgency/Frequency (PUF) patient symptom scale, Female Genitourinary Pain Index (F-GUPI), and the Short Form-36 (SF-36) Quality of Life questionnaires, completed at weeks 1 (baseline), 4 (conclusion), and 12 (8-week follow-up), were used to assess secondary outcomes. A 7-day voiding diary was collected at baseline and 4 weeks. Treatment effects were assessed via Wilcoxon-signed rank test; p<0.05 = significant.
Results: Of 10 participants, 8 completed the 4-week treatment, and 7/8 (87.5%) had a significant reduction in pelvic pain (6.88 to 4.19, p=0.011) after 4 weeks. There was also a significant decrease in ICSI, ICPI, PUF, and GUPI symptom scores, daily number of voids, and mean nocturia score (Table 1, p<0.05). Patients also experienced significant increases in the SF-36 quality of life energy/fatigue, social function, and pain scores (P <0.05). At 8-week post-therapy, the positive effects were somewhat attenuated, yet 3/7 patients (42.9%) continued to have significant pain reduction (p=0.047). Mean NRS, ICSI, and PUF scores remained significantly lower (p < 0.05), and the SF-36 energy/fatigue remained significantly elevated while emotional well-being and general health scores (unchanged at 4 weeks) had increased significantly from baseline (p < 0.05). No adverse events were reported.
Conclusions: This proof-of-concept interventional trial found significant pain reduction, decreases in subjective symptom scores, and increases in quality-of-life scores for nearly all patients. Many benefits persisted for 8 weeks post-therapy. Randomized, sham-controlled trials are necessary to validate these findings.
Source of Funding: N/a
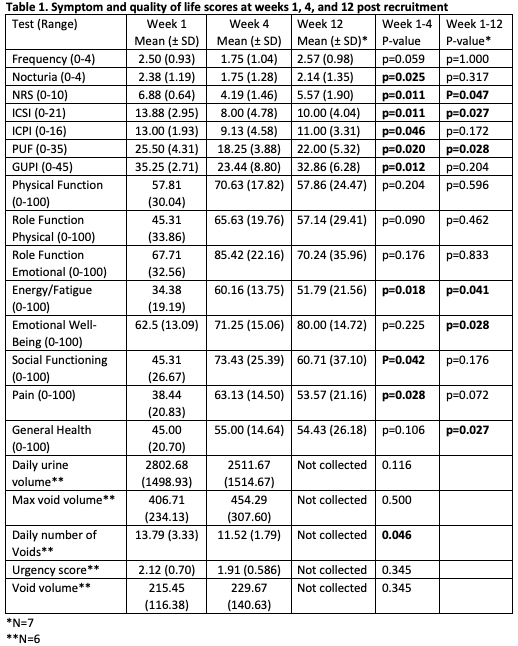
Methods: Women (ages 18-80 yo) with a confirmed diagnosis of IC/BPS, a numeric rating scale (NRS) score for pelvic pain = 6, and an anesthetic bladder capacity (BC) >400ml underwent 8-minute full body PEMF sessions, twice daily, for 4 weeks. The primary outcome measure was a reduction in NRS score = 2-points. The O’Leary Sant Interstitial Cystitis Symptom and Problem Indices (ICSI/ICPI), Pain and Urgency/Frequency (PUF) patient symptom scale, Female Genitourinary Pain Index (F-GUPI), and the Short Form-36 (SF-36) Quality of Life questionnaires, completed at weeks 1 (baseline), 4 (conclusion), and 12 (8-week follow-up), were used to assess secondary outcomes. A 7-day voiding diary was collected at baseline and 4 weeks. Treatment effects were assessed via Wilcoxon-signed rank test; p<0.05 = significant.
Results: Of 10 participants, 8 completed the 4-week treatment, and 7/8 (87.5%) had a significant reduction in pelvic pain (6.88 to 4.19, p=0.011) after 4 weeks. There was also a significant decrease in ICSI, ICPI, PUF, and GUPI symptom scores, daily number of voids, and mean nocturia score (Table 1, p<0.05). Patients also experienced significant increases in the SF-36 quality of life energy/fatigue, social function, and pain scores (P <0.05). At 8-week post-therapy, the positive effects were somewhat attenuated, yet 3/7 patients (42.9%) continued to have significant pain reduction (p=0.047). Mean NRS, ICSI, and PUF scores remained significantly lower (p < 0.05), and the SF-36 energy/fatigue remained significantly elevated while emotional well-being and general health scores (unchanged at 4 weeks) had increased significantly from baseline (p < 0.05). No adverse events were reported.
Conclusions: This proof-of-concept interventional trial found significant pain reduction, decreases in subjective symptom scores, and increases in quality-of-life scores for nearly all patients. Many benefits persisted for 8 weeks post-therapy. Randomized, sham-controlled trials are necessary to validate these findings.
Source of Funding: N/a


.jpg)
.jpg)