Back
Poster, Podium & Video Sessions
Moderated Poster
MP18: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Non-neurogenic Voiding Dysfunction I
MP18-10: Evaluation of clinical performance and safety for the rechargeable InterStimTM Micro in Overactive Bladder Subjects: 3-month results from the Global Post-Market ELITE study
Friday, May 13, 2022
4:30 PM – 5:45 PM
Location: Room 222
Keith Xavier, Arlington , TX, Colin Goudelocke*, New Orleans, LA, Barry Pecha, Jeffersonville, IN, Kimberly Burgess, Greenville, SC, Marie-Aimée Perrouin-Verbe, Nantes, France, Ryan Krlin, New Orleans, LA, Jodi Michaels, Woodbury, MN, Sagar Shah, Jacksonville, FL, Benoît Peyronnet, Rennes, France, Stanley Zaslau, Morgantown, WV, Bianca Papi, Katie Bittner, Minneapolis, MN, Dean Elterman, Toronto, Canada, Victor Nitti, Los Angeles, CA

Colin Murrah Goudelocke, MD
Ochsner Medical Center
Poster Presenter(s)
Introduction: Sacral Neuromodulation (SNM) is an established advanced therapy for treatment of symptoms of overactive bladder, nonobstructive urinary retention, and fecal incontinence supported by studies with long-term follow up. The ELITE study is an ongoing global, prospective, post-market study to confirm the clinical performance and safety of the InterStimTM Micro SNM system in all indicated conditions.
Methods: Eligible subjects with symptoms of OAB (greater than or equal to 8 urgency frequency episodes per day and/or 3 or more episodes of urinary urge incontinence on a 3-day voiding diary) were enrolled in the OAB cohort following successful therapy evaluation and neurostimulator implant. Subjects completed voiding diaries and questionnaires at baseline and 3-month follow up. Safety was evaluated by collection of reportable adverse events (AE). Data are reported as mean ± standard deviation or with 95% confidence intervals (CI).
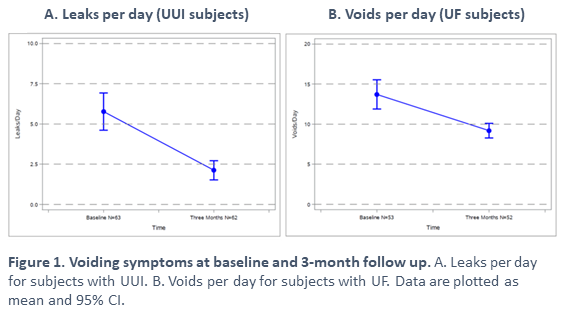
Results: Subjects enrolled in the OAB cohort (n=68) had a primary diagnosis of UUI (22%), UF (7%), or both UUI and UF (71%). Most subjects were female (89.7%). The mean age was 62±13 years and BMI was 32±7 kg/m2. At baseline UUI subjects (n=63) had 6.0±4.5 leaks per day and UF subjects (n=53) had 14±6.5 voids per day. At the 3-month follow up, the average change in leaks per day was -4.0 (95% CI: -4.7, -2.6) for UUI subjects (n=62) and average change in voids per day was -5.0 (95% CI: -6.3, -2.7) for UF subjects (n=62). The average degree of urgency was 2 ± 0.5 for UUI subjects and 2 ± 0.6 for UF subjects at baseline on a 0-4 scale. At 3-month follow up, urgency was 1.4 ± 0.6 and 1.4 ± 0.7 for UUI and UF subjects, respectively. The incidence of device-, procedure-, or therapy- related AEs in enrolled subjects was 2.9% (2/68) and serious AEs was 1.5% (1/68) at the time of database snapshot. There were no unanticipated adverse device effects.
Conclusions: The change in symptoms is consistent with previous reports and rate of adverse events is favorable compared to previously reported rates for SNM thereby confirming the safety and clinical performance of InterStimTM Micro for subjects with OAB. These data add to the established SNM evidence with data from the rechargeable InterStimTM Micro.
Source of Funding: Medtronic
Methods: Eligible subjects with symptoms of OAB (greater than or equal to 8 urgency frequency episodes per day and/or 3 or more episodes of urinary urge incontinence on a 3-day voiding diary) were enrolled in the OAB cohort following successful therapy evaluation and neurostimulator implant. Subjects completed voiding diaries and questionnaires at baseline and 3-month follow up. Safety was evaluated by collection of reportable adverse events (AE). Data are reported as mean ± standard deviation or with 95% confidence intervals (CI).
Results: Subjects enrolled in the OAB cohort (n=68) had a primary diagnosis of UUI (22%), UF (7%), or both UUI and UF (71%). Most subjects were female (89.7%). The mean age was 62±13 years and BMI was 32±7 kg/m2. At baseline UUI subjects (n=63) had 6.0±4.5 leaks per day and UF subjects (n=53) had 14±6.5 voids per day. At the 3-month follow up, the average change in leaks per day was -4.0 (95% CI: -4.7, -2.6) for UUI subjects (n=62) and average change in voids per day was -5.0 (95% CI: -6.3, -2.7) for UF subjects (n=62). The average degree of urgency was 2 ± 0.5 for UUI subjects and 2 ± 0.6 for UF subjects at baseline on a 0-4 scale. At 3-month follow up, urgency was 1.4 ± 0.6 and 1.4 ± 0.7 for UUI and UF subjects, respectively. The incidence of device-, procedure-, or therapy- related AEs in enrolled subjects was 2.9% (2/68) and serious AEs was 1.5% (1/68) at the time of database snapshot. There were no unanticipated adverse device effects.
Conclusions: The change in symptoms is consistent with previous reports and rate of adverse events is favorable compared to previously reported rates for SNM thereby confirming the safety and clinical performance of InterStimTM Micro for subjects with OAB. These data add to the established SNM evidence with data from the rechargeable InterStimTM Micro.
Source of Funding: Medtronic


.jpg)
.jpg)