Back
Poster, Podium & Video Sessions
Moderated Poster
MP23: Bladder Cancer: Invasive III
MP23-18: Preliminary results of a urine-based DNA methylation test to monitor response to neoadjuvant therapy in muscle-invasive bladder cancer
Saturday, May 14, 2022
8:45 AM – 10:00 AM
Location: Room 225
Seyedeh Sanam Ladi Seyedian*, Los Angeles, CA, Saum Ghodoussipour, New Brunswick, CA, Hamed Ahmadi, Ryan Lee, Alireza Ghoreifi, Michael Basin, Los Angeles, CA, Paolo Piatti, Taikun Yamada, Benjamin Jara, Lucy Sanossian, Irvine, CA, Hooman Djaladat, Anne Schuckman, Sumeet Bhanvadia, Gangning Liang, Siamak Daneshmand, Los Angeles, CA

Seyedeh Sanam Ladi Seyedian, MD
University of Southern California
Poster Presenter(s)
Introduction: Neoadjuvant chemotherapy provides a survival benefit compared to cystectomy alone in muscle invasive bladder cancer (MIBC), but only in those who respond at least partially to the treatment. Herein, we explore the feasibility of a urine-based DNA methylation test to monitor response to neoadjuvant therapy (NAT) in the bladder.
Methods: Urine samples were collected from MIBC patients undergoing NAT (chemotherapy or immunotherapy) under an IRB approved protocol at baseline and after each therapy cycle. Samples were analyzed with Bladder CARE (Pangea Laboratory), a urine-based assay that measures methylation levels of 3 bladder-cancer specific biomarkers (TRNA-Cys, SIM2, and NKX1-1) and two internal control loci using methylation-sensitive restriction enzymes coupled with qPCR. Results are reported as Bladder CARE Index (BCI) score and categorized as “positive”, “high-risk”, or “negative” based on previously validated segregation of data. Changes in BCI score and surgical pathology were reviewed to determine association.
Results: Of 45 enrolled patients, 30 completed NAT and underwent cystectomy. Gemcitabine/Cisplatin (GemCis) was used in 19/30 patients (Table 1). A decrease in BCI was seen in 19/30 (63%) patients. Of the patients with a decrease in BCI, 14 (74%) showed at least partial response to NAT. Eleven patients had increase or no change in BCI, of which, 8 patients had residual disease at cystectomy. Four patients with complete response on final pathology had a negative BCI at baseline due to prior complete transurethral resection of the tumor and throughout NAT (#7, 17, 23, 31). Final BCI was correlated with final pathology in 24/30 (80%) patients. Seventeen patients had positive/high risk BCI and residual disease at the time of cystectomy and 7 patients had negative BCI and no residual disease at the time of cystectomy.
Conclusions: We present a novel urine based epigenetic assay that may have utility in monitoring response to NAT in the bladder in patients with MIBC. Further studies are underway to evaluate the utility of this test.
Source of Funding: Zymo Research Corp, Pangea Laboratory
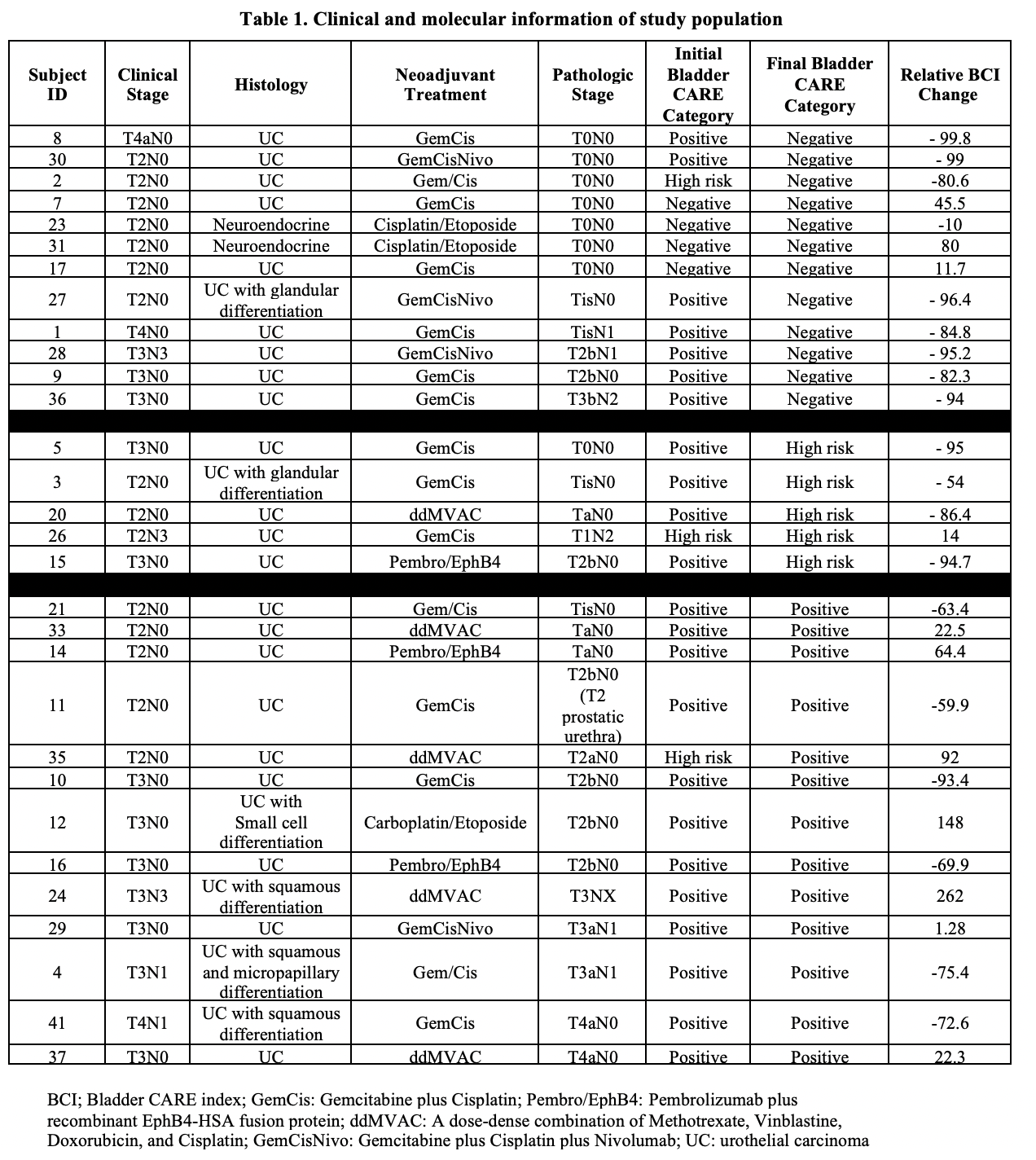
Methods: Urine samples were collected from MIBC patients undergoing NAT (chemotherapy or immunotherapy) under an IRB approved protocol at baseline and after each therapy cycle. Samples were analyzed with Bladder CARE (Pangea Laboratory), a urine-based assay that measures methylation levels of 3 bladder-cancer specific biomarkers (TRNA-Cys, SIM2, and NKX1-1) and two internal control loci using methylation-sensitive restriction enzymes coupled with qPCR. Results are reported as Bladder CARE Index (BCI) score and categorized as “positive”, “high-risk”, or “negative” based on previously validated segregation of data. Changes in BCI score and surgical pathology were reviewed to determine association.
Results: Of 45 enrolled patients, 30 completed NAT and underwent cystectomy. Gemcitabine/Cisplatin (GemCis) was used in 19/30 patients (Table 1). A decrease in BCI was seen in 19/30 (63%) patients. Of the patients with a decrease in BCI, 14 (74%) showed at least partial response to NAT. Eleven patients had increase or no change in BCI, of which, 8 patients had residual disease at cystectomy. Four patients with complete response on final pathology had a negative BCI at baseline due to prior complete transurethral resection of the tumor and throughout NAT (#7, 17, 23, 31). Final BCI was correlated with final pathology in 24/30 (80%) patients. Seventeen patients had positive/high risk BCI and residual disease at the time of cystectomy and 7 patients had negative BCI and no residual disease at the time of cystectomy.
Conclusions: We present a novel urine based epigenetic assay that may have utility in monitoring response to NAT in the bladder in patients with MIBC. Further studies are underway to evaluate the utility of this test.
Source of Funding: Zymo Research Corp, Pangea Laboratory


.jpg)
.jpg)