Back
Poster, Podium & Video Sessions
Moderated Poster
MP25: Trauma/Reconstruction/Diversion: Ureter (including Pyeloplasty) and Bladder Reconstruction (including fistula), Augmentation, Substitution, Diversion
MP25-18: Separate Abdominal Closure Trays Significantly Improve Cystectomy Surgical Site Infection; Outcomes from a pre-post Implementation National Surgical Quality Inpatient Program (NSQIP) Project
Saturday, May 14, 2022
10:30 AM – 11:45 AM
Location: Room 228
Kevin Krughoff*, Christopher Mantyh, Charles D Scales, Andrew C. Peterson, Durham, NC

Kevin Krughoff, MD
Duke University
Poster Presenter(s)
Introduction: While surgical site infection (SSI) prevention bundles have gained popularity across many disciplines, the urologic experience remains underreported. Our goal was to assess the impact of an abdominal closure protocol (ACP) on the incidence of SSI, UTI and procedure duration for cystectomy with urinary diversion.
Methods: A standardized abdominal closure protocol (ACP) was adopted at our institution in 2021 which included re-prep and re-drape of the abdomen, change of surgeon gowns and gloves and utilization of a separate abdominal closure tray. Utilizing the National Surgical Quality Inpatient Program (NSQIP) database, patients who underwent cystectomy and urinary diversion between 2016-2021 were identified for assessment. Cases incorporating the ACP were matched to cases pre-dating the incorporation of the ACP through propensity score matching based on 16 NSQIP-derived predictor variables. The optimal matching algorithm was determined based on greatest resulting reductions in standardized mean differences and variance ratios between groups. Counterfactual outcomes were generated using logistic regression to determine the average treatment effect of ACP incorporation. Standard errors and the 95% confidence intervals were calculated from 1,000 bootstrap samples.
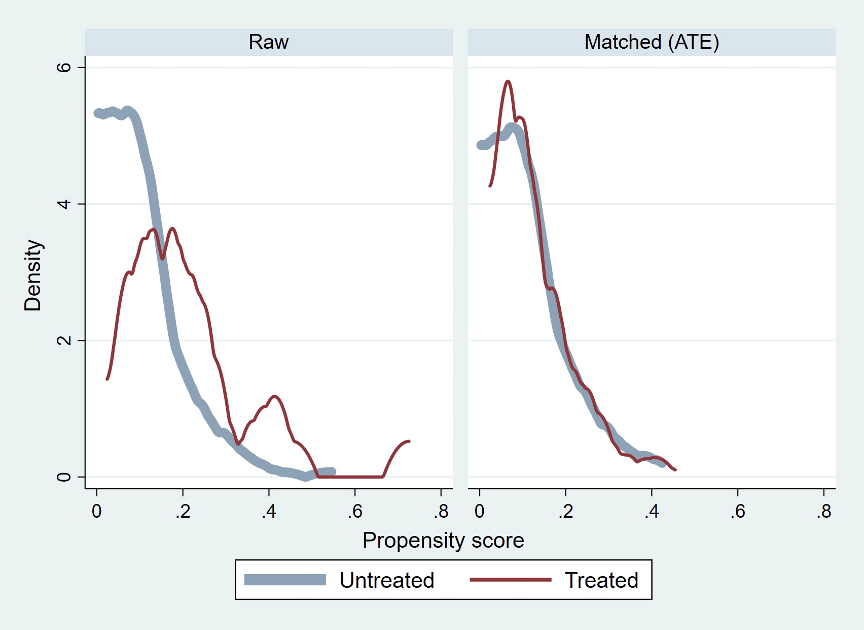
Results: A total of 327 cases at our facility were reviewed. Twenty-three of 24 ACP cases were successfully matched to 245 of 303 non-ACP cases using a kernel-matching algorithm (Figure 1). Matching resulted in standardized mean differences below 0.1 for 14 of 16 NSQIP predictor variables and variance ratios of less than 2.0 for all NSQIP predictor variables. Following ACP incorporation, there was a raw SSI reduction of 5.7%, a raw UTI reduction of 5.6% and a raw increase in procedure duration of 38.4 minutes. The estimated treatment effect after modeling was a 4.1% reduction in SSI (p < 0.02, CI 0.6% to 7.5%), with no significant effect on UTI incidence or procedure duration.
Conclusions: Using a treatment effects model, the incorporation of an abdominal closure protocol was associated with a significant reduction in surgical site infection with minimal increase in surgical time.
Source of Funding: Boston Scientific Fellowship Grant
Methods: A standardized abdominal closure protocol (ACP) was adopted at our institution in 2021 which included re-prep and re-drape of the abdomen, change of surgeon gowns and gloves and utilization of a separate abdominal closure tray. Utilizing the National Surgical Quality Inpatient Program (NSQIP) database, patients who underwent cystectomy and urinary diversion between 2016-2021 were identified for assessment. Cases incorporating the ACP were matched to cases pre-dating the incorporation of the ACP through propensity score matching based on 16 NSQIP-derived predictor variables. The optimal matching algorithm was determined based on greatest resulting reductions in standardized mean differences and variance ratios between groups. Counterfactual outcomes were generated using logistic regression to determine the average treatment effect of ACP incorporation. Standard errors and the 95% confidence intervals were calculated from 1,000 bootstrap samples.
Results: A total of 327 cases at our facility were reviewed. Twenty-three of 24 ACP cases were successfully matched to 245 of 303 non-ACP cases using a kernel-matching algorithm (Figure 1). Matching resulted in standardized mean differences below 0.1 for 14 of 16 NSQIP predictor variables and variance ratios of less than 2.0 for all NSQIP predictor variables. Following ACP incorporation, there was a raw SSI reduction of 5.7%, a raw UTI reduction of 5.6% and a raw increase in procedure duration of 38.4 minutes. The estimated treatment effect after modeling was a 4.1% reduction in SSI (p < 0.02, CI 0.6% to 7.5%), with no significant effect on UTI incidence or procedure duration.
Conclusions: Using a treatment effects model, the incorporation of an abdominal closure protocol was associated with a significant reduction in surgical site infection with minimal increase in surgical time.
Source of Funding: Boston Scientific Fellowship Grant


.jpg)
.jpg)