Back
Poster, Podium & Video Sessions
Moderated Poster
MP27: Prostate Cancer: Advanced (including Drug Therapy) I
MP27-01: Time on treatment with abiraterone in men with de novo metastatic castration sensitive prostate cancer
Saturday, May 14, 2022
10:30 AM – 11:45 AM
Location: Room 222
Pär Stattin*, Hans Garmo, Uppsala, Sweden, Camilla Thellenberg-Karlsson, Umeå, Sweden, Giuseppe Fallara, Milan, Italy, Johan Styrke, Umeå, Sweden, Ingela Franck Lissbrant, Gothenburg, Sweden, Rolf Gedeborg, Uppsala, Sweden
- PS
Poster Presenter(s)
Introduction: Patient selection and time on treatment with abiraterone for de novo metastatic castration sensitive prostate cancer (mCSPC) may be different in clinical practice compared to randomised clinical trials, since men in RCTs are generally younger and fitter than men treated in clinical practise. However, to date there are no data on time on treatment with abiraterone in men with de novo mCSPC from clinical practise.
Methods: In this nation-wide population-based study, The National Prostate Cancer Register of Sweden was linked to the Patient Register and the Prescribed Drug Register. We estimated time on treatment with abiraterone in men with de novo mCSPC who had been diagnosed between 1 June 2018 and 31 December 2019. The cumulative incidence of treatment discontinuation was calculated considering death as competing risk.
Results: Out of 1511 men with de novo mCSPC diagnosed during the study period, 12% initiated treatment with abiraterone within 6 months from prostate cancer diagnosis. These men were younger, had higher Gleason score, higher prostate-specific antigen, but less comorbidity, compared to men with de novo mCSPC not treated with abiraterone. After a median follow-up of 14 months, the median time on treatment with abiraterone was 14 months, which is substantially shorter compared to median time on treatment of 26 -33 months reported in RCTs. The shorter time on treatment did not appear to be due to high mortality.
Conclusions: During the early post marketing period, the time on treatment with abiraterone for de novo metastatic castration sensitive prostate cancer was almost two-fold shorter compared to what was reported from RCTs. Further studies are warranted to investigate the reasons for discontinuation of abiraterone and to investigate the consequences of the shorter treatment time on outcome.
Source of Funding: Swedish Cancer Society
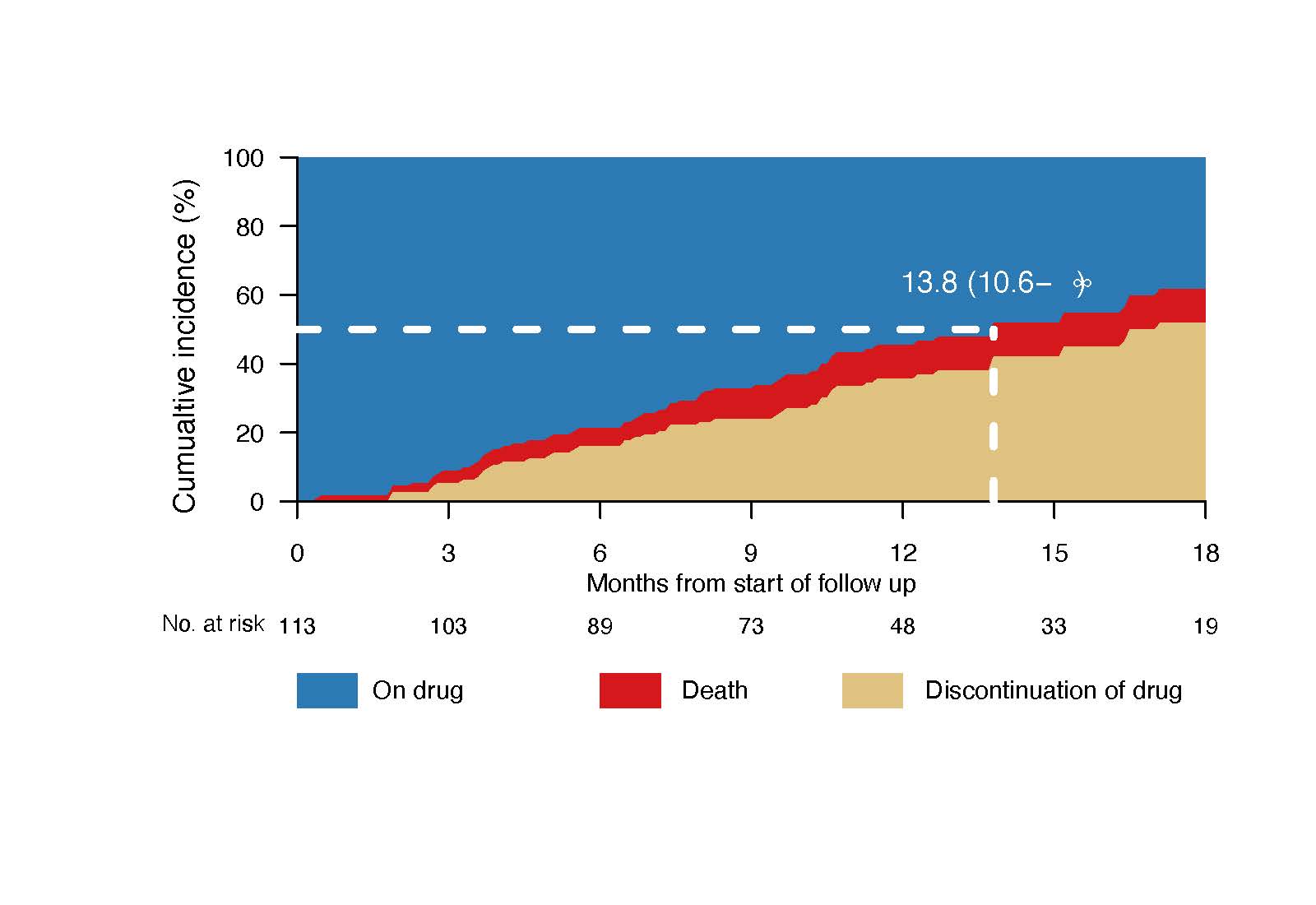
Methods: In this nation-wide population-based study, The National Prostate Cancer Register of Sweden was linked to the Patient Register and the Prescribed Drug Register. We estimated time on treatment with abiraterone in men with de novo mCSPC who had been diagnosed between 1 June 2018 and 31 December 2019. The cumulative incidence of treatment discontinuation was calculated considering death as competing risk.
Results: Out of 1511 men with de novo mCSPC diagnosed during the study period, 12% initiated treatment with abiraterone within 6 months from prostate cancer diagnosis. These men were younger, had higher Gleason score, higher prostate-specific antigen, but less comorbidity, compared to men with de novo mCSPC not treated with abiraterone. After a median follow-up of 14 months, the median time on treatment with abiraterone was 14 months, which is substantially shorter compared to median time on treatment of 26 -33 months reported in RCTs. The shorter time on treatment did not appear to be due to high mortality.
Conclusions: During the early post marketing period, the time on treatment with abiraterone for de novo metastatic castration sensitive prostate cancer was almost two-fold shorter compared to what was reported from RCTs. Further studies are warranted to investigate the reasons for discontinuation of abiraterone and to investigate the consequences of the shorter treatment time on outcome.
Source of Funding: Swedish Cancer Society


.jpg)
.jpg)