Back
Poster, Podium & Video Sessions
Moderated Poster
MP27: Prostate Cancer: Advanced (including Drug Therapy) I
MP27-10: Clinical utility of next-generation sequencing for prostate cancer in the context of a changing treatment landscape
Saturday, May 14, 2022
10:30 AM – 11:45 AM
Location: Room 222
Jacqueline R. Griffin, MS, PhD*, Tomi Jun, MD, Sunny Guin, PhD, Stamford, CT, Bobby Chi-Hung Liaw, MD, Vaibhav G. Patel, MD, Brooklyn, NY, Che-Kai Tsao, MD, Astoria, NY, Michael R. Rossi, PhD, Rong Chen, PhD, Xiang Zhou, PhD, Feras Hantash, MD, MS, PhD, Stamford, CT, William K. Oh, MD, New York, NY
Poster Presenter(s)
Introduction: The clinical utility of next-generation sequencing (NGS) depends on the availability of sequencing-directed therapies (SDT). There were no FDA-approved SDTs in prostate cancer (PCa) until 2020, when PARP inhibitors olaparib and rucaparib were approved for tumors bearing homologous recombination repair (HRR) genes. We assessed the clinical utility of NGS in PCa before and after the approval of these agents in a single academic medical center.
Methods: For PCa patients seen at Mount Sinai Hospital (New York, NY: 2018–2021) receiving NGS (161-gene Sema4 Signal Solid Tumor Panel), data were extracted from Mount Sinai electronic medical record using a proprietary automated pipeline with limited manual curation (Sema4 PRODB). The primary outcome was clinical utility in metastatic PCa, defined as proportion of metastatic PCa patients receiving SDT. Secondary outcomes included time-to-next-treatment (TTNT, time from SDT start to the start of next systemic therapy) and the proportion of patients with clinically actionable (as of 9/2021) alterations, defined as either Tier 1 (FDA-approved treatments in prostate cancer) or Tier 2 (either off-label or investigational agents).
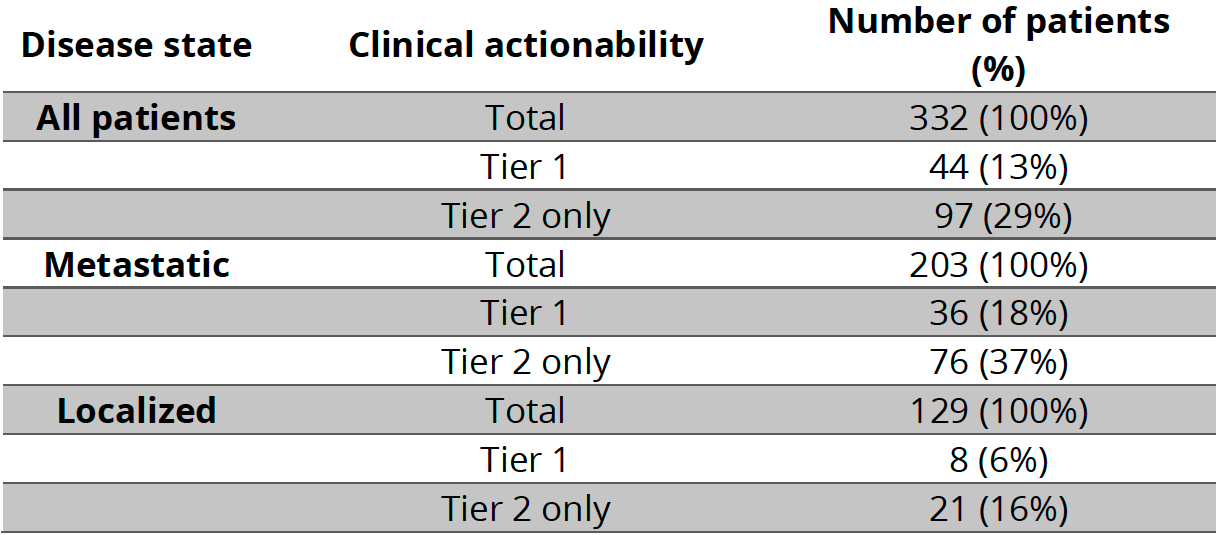
Results: Of 332 PCa patients; 51% (N=170) were sequenced 2020 or later. Median age at diagnosis was 65 (IQR 12). Of which, 39% (N=129) were localized and 61% (N=203) metastatic. We identified 167 actionable alterations in 125 patients (38% of cohort). Of actionable alterations, 31% (N=51) were Tier 1 and 69% (N=116) Tier 2. Of the 44 patients with Tier 1 alterations, 8 (18%) received SDT (all received olaparib). Proportion of metastatic patients receiving olaparib increased from 1% (2/145) before 2020 to 10% (6/58) during or after 2020 (p=0.008). Of the 36 patients not receiving olaparib: 20 were sequenced before FDA approval and treated with an alternative systemic therapy; 8 had localized disease and were not eligible; 8 had limited follow-up or unknown treatment status. For those who received olaparib, the median TTNT was 5 months.
Conclusions: Clinical utility of NGS was linked to treatment landscape. Increases in NGS test volume and olaparib use coincided with approval of PARP inhibitors for HRR-mutated PCa patients. Notably, NGS was used to match patients to off-label/investigational olaparib before its FDA approval.
Source of Funding: Sema4
Methods: For PCa patients seen at Mount Sinai Hospital (New York, NY: 2018–2021) receiving NGS (161-gene Sema4 Signal Solid Tumor Panel), data were extracted from Mount Sinai electronic medical record using a proprietary automated pipeline with limited manual curation (Sema4 PRODB). The primary outcome was clinical utility in metastatic PCa, defined as proportion of metastatic PCa patients receiving SDT. Secondary outcomes included time-to-next-treatment (TTNT, time from SDT start to the start of next systemic therapy) and the proportion of patients with clinically actionable (as of 9/2021) alterations, defined as either Tier 1 (FDA-approved treatments in prostate cancer) or Tier 2 (either off-label or investigational agents).
Results: Of 332 PCa patients; 51% (N=170) were sequenced 2020 or later. Median age at diagnosis was 65 (IQR 12). Of which, 39% (N=129) were localized and 61% (N=203) metastatic. We identified 167 actionable alterations in 125 patients (38% of cohort). Of actionable alterations, 31% (N=51) were Tier 1 and 69% (N=116) Tier 2. Of the 44 patients with Tier 1 alterations, 8 (18%) received SDT (all received olaparib). Proportion of metastatic patients receiving olaparib increased from 1% (2/145) before 2020 to 10% (6/58) during or after 2020 (p=0.008). Of the 36 patients not receiving olaparib: 20 were sequenced before FDA approval and treated with an alternative systemic therapy; 8 had localized disease and were not eligible; 8 had limited follow-up or unknown treatment status. For those who received olaparib, the median TTNT was 5 months.
Conclusions: Clinical utility of NGS was linked to treatment landscape. Increases in NGS test volume and olaparib use coincided with approval of PARP inhibitors for HRR-mutated PCa patients. Notably, NGS was used to match patients to off-label/investigational olaparib before its FDA approval.
Source of Funding: Sema4


.jpg)
.jpg)
