Back
Poster, Podium & Video Sessions
Moderated Poster
MP27: Prostate Cancer: Advanced (including Drug Therapy) I
MP27-16: Oral Relugolix for Androgen Deprivation Therapy in Advanced Prostate Cancer: Detailed Safety Analysis from the Randomized Phase 3 HERO Study
Saturday, May 14, 2022
10:30 AM – 11:45 AM
Location: Room 222
Bryan Mehlhaff*, Springfield, OR, Neal D. Shore, Myrtle Beach, SC, Daniel J. George, Durham, NC, Michael S. Cookson, Oklahoma City, OK, Daniel R. Saltzstein, San Antonio, TX, Ronald Tutrone, Towson, MD, James L. Bailen, Jeffersonville, IN, Bruce Brown, Andria G.M. Langenberg, Mark Fallick, Sophia Lu, Brisbane, CA, Sarah Hanson, New York, NY, Bertrand Tombal, Brussels, Belgium, Fred Saad, Montreal, Canada
- NS
Neal D. Shore, MD
Carolina Urologic Research Center
Poster Presenter(s)
Introduction: In the HERO study in men with advanced prostate cancer, relugolix, a highly selective nonpeptide oral GnRH antagonist, was well tolerated, with hot flash and fatigue as the most frequently reported adverse events (AE) for men in both relugolix and leuprolide groups. A 54% lower risk of major adverse cardiovascular events (MACE) was observed for men on relugolix vs leuprolide. Herein, we provide a detailed analysis of the safety results from the HERO study, including reviewing AE onset and duration data.
Methods: The phase 3 HERO study evaluated 930 men with advanced prostate cancer who were randomized 2:1 and treated with relugolix 120 mg orally once daily (after a 360 mg day 1 loading dose) or leuprolide injections every 12 weeks for 48 weeks. Safety assessments included AEs (assessed according to the National Cancer Institute Common Terminology Criteria for AEs, version 4.03), MACE (defined as nonfatal myocardial infarction, non-fatal stroke, and death from any cause), as well as onset (median days from the date of first dose to the initial event) and duration (median days from start to end date of the event) of the most common events.
Results: AEs were reported in 92.9% of men in relugolix group and 93.5% in the leuprolide group, with hot flash, fatigue, constipation, diarrhea, and arthralgia occurring most frequently (table). Grade =3 AEs were reported in 18.0% in the relugolix group and 20.5% men in the leuprolide group. The most frequently reported (=1%) grade = 3 AEs in any treatment group included hypertension, diabetes mellitus, and syncope. All other grade = 3 AEs were reported with similar incidence in both treatment groups. MACE occurred in 2.9% and 6.2% of patients on relugolix and leuprolide, respectively. For AEs occurring in = 10% patients, median time to onset was 19.0-142.0 days in the relugolix group and 41.0-188.5 days in the leuprolide group. Duration varied among the AEs (table).
Conclusions: Relugolix, an oral nonpeptide GnRH receptor antagonist, was generally well tolerated in the phase 3 HERO study. Results for AE onset and duration suggest AEs occur early during treatment with varying duration depending on the type of event.
Source of Funding: Myovant Sciences GmBH, in collaboration with Pfizer.
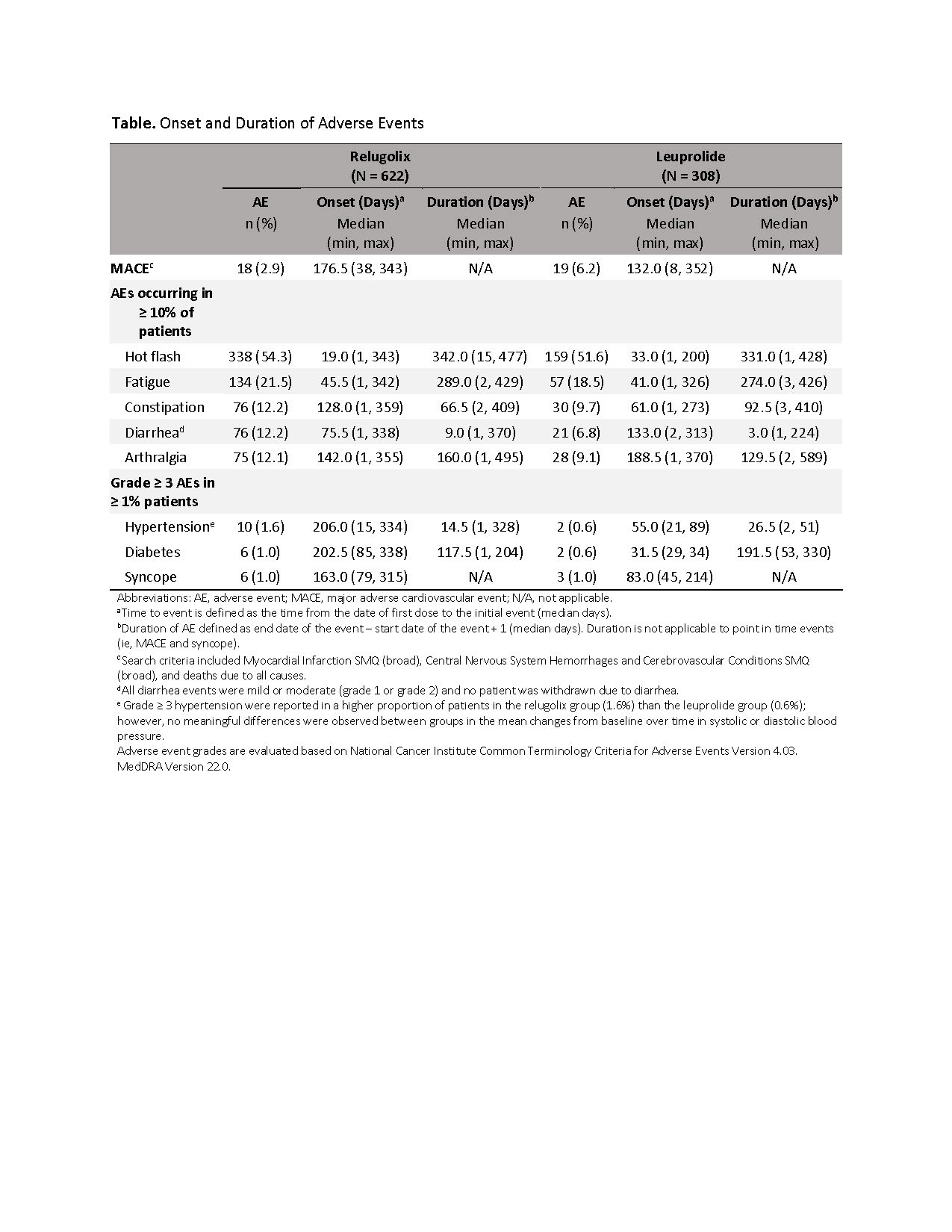
Methods: The phase 3 HERO study evaluated 930 men with advanced prostate cancer who were randomized 2:1 and treated with relugolix 120 mg orally once daily (after a 360 mg day 1 loading dose) or leuprolide injections every 12 weeks for 48 weeks. Safety assessments included AEs (assessed according to the National Cancer Institute Common Terminology Criteria for AEs, version 4.03), MACE (defined as nonfatal myocardial infarction, non-fatal stroke, and death from any cause), as well as onset (median days from the date of first dose to the initial event) and duration (median days from start to end date of the event) of the most common events.
Results: AEs were reported in 92.9% of men in relugolix group and 93.5% in the leuprolide group, with hot flash, fatigue, constipation, diarrhea, and arthralgia occurring most frequently (table). Grade =3 AEs were reported in 18.0% in the relugolix group and 20.5% men in the leuprolide group. The most frequently reported (=1%) grade = 3 AEs in any treatment group included hypertension, diabetes mellitus, and syncope. All other grade = 3 AEs were reported with similar incidence in both treatment groups. MACE occurred in 2.9% and 6.2% of patients on relugolix and leuprolide, respectively. For AEs occurring in = 10% patients, median time to onset was 19.0-142.0 days in the relugolix group and 41.0-188.5 days in the leuprolide group. Duration varied among the AEs (table).
Conclusions: Relugolix, an oral nonpeptide GnRH receptor antagonist, was generally well tolerated in the phase 3 HERO study. Results for AE onset and duration suggest AEs occur early during treatment with varying duration depending on the type of event.
Source of Funding: Myovant Sciences GmBH, in collaboration with Pfizer.


.jpg)
.jpg)