Back
Poster, Podium & Video Sessions
Moderated Poster
MP31: Health Services Research: Quality Improvement & Patient Safety II
MP31-05: Large scale implementation of opioid prescription reduction after robotic prostatectomy – 2 year evaluation from the Pennsylvania Urologic Regional Collaborative (PURC)
Saturday, May 14, 2022
2:45 PM – 4:00 PM
Location: Room 228
Thenappan Chandrasekar*, Philadelphia, PA, Necole Streeper, Charles Keith, Hershey, PA, Andrea Quin, Kaynaat Syed, Alexander Kutikov, Philadelphia, PA, John Danella, Danville, PA, Thomas Lanchoney, Paoli, PA, Jeffrey Tomaszewski, Camden, NJ, Edouard Trabulsi, Adam Reese, Marc Smaldone, Robert Uzzo, Thomas Guzzo, Philadelphia, PA, Jay Raman, Hershey, PA, Adrien Bernstein, Daniel Lee, Philadelphia, PA

Thenappan Chandrasekar, MD
Assistant Professor
Thomas Jefferson University
Poster Presenter(s)
Introduction: Diversion of post-operative medication is a major contributor to opioid misuse. The objective of this study is to evaluate the impact of implementing an opioid reduction protocol for robotic prostatectomy (RALP) within a regional collaborative.
Methods: An opioid reduction protocol was implemented for patients undergoing RALP at four institutions within the Pennsylvania Urologic Regional Collaborative (PURC). We compared prescribing practices 12 months before and after the intervention, with a one-month washout period. We measured opioid inpatient orders and prescriptions on discharge and patient reported pain levels at their first post-operative visit. All opioid prescriptions were converted into equivalents of oxycodone 5mg tablets. Pain scores were captured with a visual analog scale of 0-10.
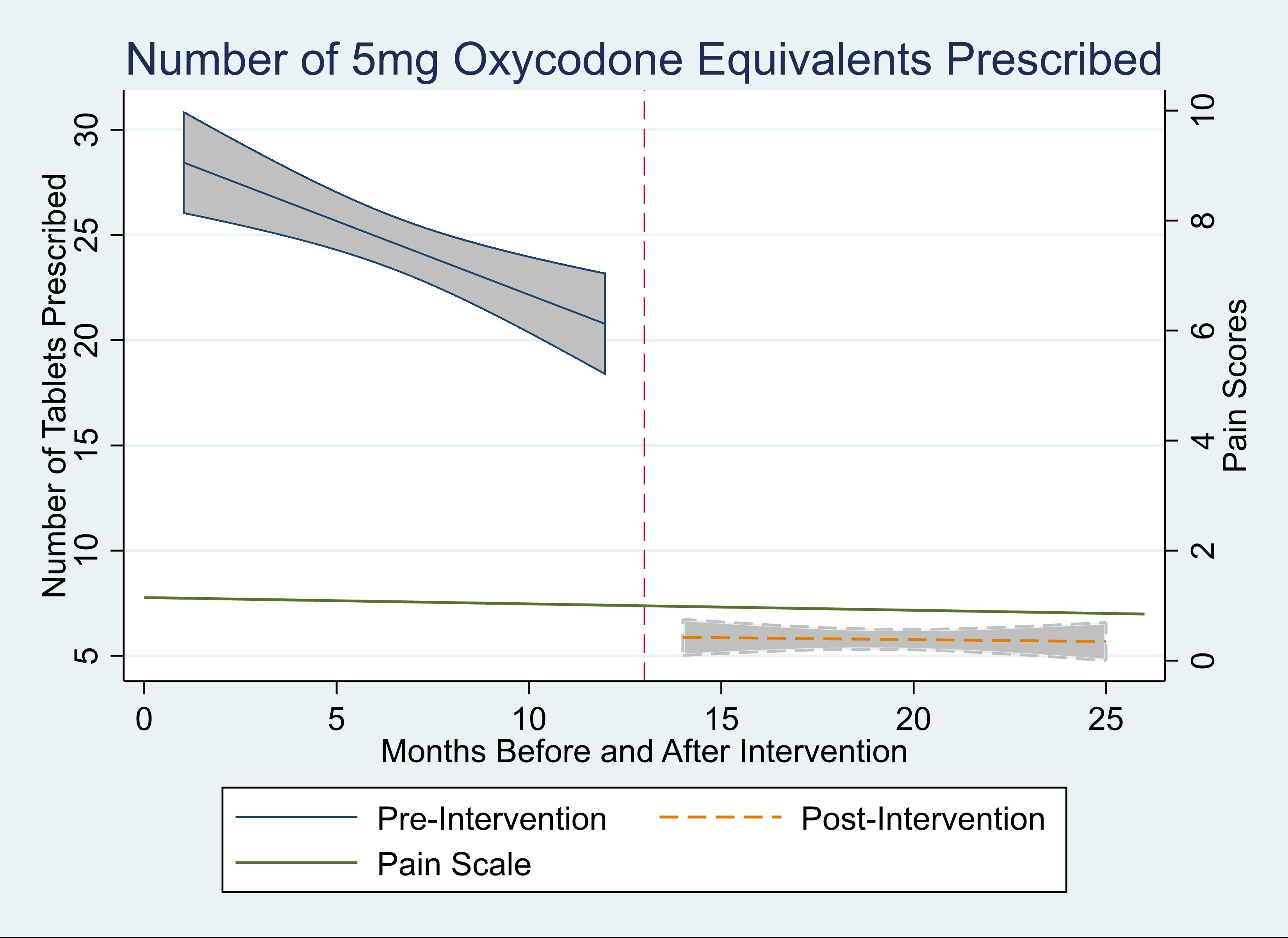
Results: Overall, 2,061 patients underwent RALP. For the 12 months before implementation, a median of 2.7 (IQR 1.3-4.5) oxycodone 5mg tablets were prescribed as an inpatient, and 20 tablets were prescribed on discharge (IQR 14-30). After implementation, this decreased to a median of 2.2 (IQR 0.2-4.1) tablets as an inpatient, and median of 0 tablets (IQR 0-14, p<0.001) prescribed. The percentage of patients who had no opioid use as inpatients but were still given opioids on discharge decreased from 98% before implementation to 25% after implementation (p < 0.001). Despite the decrease in opioid prescriptions, there were no significant changes in pain scores (median value 0/10, p=0.78, Fig. 1). On average, 14 less opioid tablets were prescribed per RALP, leading to a total of 14,582 less opioid tablets in Pennsylvania over a 1-year period.
Conclusions: Adequate pain control is feasible without opiates for the majority of patients undergoing RALP, allowing for significant reductions in the number of opioids entering the community. Policies to encourage opioid stewardship should be designed to encourage adoption of such programmatic change.
Source of Funding: Data was provided with permission from the Pennsylvania Urology Regional Collaborative (PURC), funded by participating urology practices and the Partnership for Patient Care, a quality improvement initiative supported by the Health Care Improvement Foundation, Independence Blue Cross, and southeastern PA hospitals and health systems.
Methods: An opioid reduction protocol was implemented for patients undergoing RALP at four institutions within the Pennsylvania Urologic Regional Collaborative (PURC). We compared prescribing practices 12 months before and after the intervention, with a one-month washout period. We measured opioid inpatient orders and prescriptions on discharge and patient reported pain levels at their first post-operative visit. All opioid prescriptions were converted into equivalents of oxycodone 5mg tablets. Pain scores were captured with a visual analog scale of 0-10.
Results: Overall, 2,061 patients underwent RALP. For the 12 months before implementation, a median of 2.7 (IQR 1.3-4.5) oxycodone 5mg tablets were prescribed as an inpatient, and 20 tablets were prescribed on discharge (IQR 14-30). After implementation, this decreased to a median of 2.2 (IQR 0.2-4.1) tablets as an inpatient, and median of 0 tablets (IQR 0-14, p<0.001) prescribed. The percentage of patients who had no opioid use as inpatients but were still given opioids on discharge decreased from 98% before implementation to 25% after implementation (p < 0.001). Despite the decrease in opioid prescriptions, there were no significant changes in pain scores (median value 0/10, p=0.78, Fig. 1). On average, 14 less opioid tablets were prescribed per RALP, leading to a total of 14,582 less opioid tablets in Pennsylvania over a 1-year period.
Conclusions: Adequate pain control is feasible without opiates for the majority of patients undergoing RALP, allowing for significant reductions in the number of opioids entering the community. Policies to encourage opioid stewardship should be designed to encourage adoption of such programmatic change.
Source of Funding: Data was provided with permission from the Pennsylvania Urology Regional Collaborative (PURC), funded by participating urology practices and the Partnership for Patient Care, a quality improvement initiative supported by the Health Care Improvement Foundation, Independence Blue Cross, and southeastern PA hospitals and health systems.


.jpg)
.jpg)