Back
Poster, Podium & Video Sessions
Moderated Poster
MP31: Health Services Research: Quality Improvement & Patient Safety II
MP31-06: Real-Time Measurement of Patient Reported Outcomes and Opioid Use Following Urologic Procedures using Automated Text Messaging
Saturday, May 14, 2022
2:45 PM – 4:00 PM
Location: Room 228
Daniel Lee*, Anish Agarwal, Zarina Ali, Ruiying Xiong, Evan Spencer, Jessica Hemmons, Kit Delgado, Philadelphia, PA
- DL
Poster Presenter(s)
Introduction: Over-prescription of postoperative opioids remains a significant problem. Measuring real-time postoperative pain and opioid consumption over a large number of procedures can guide prescribing and shared decision making. The objective was to evaluate opioid consumption and patient-reported pain intensity following urologic procedures via automated text messaging.
Methods: This was a prospective, observational study investigating patient-reported outcomes and the quantity of opioids prescribed and consumed in the context of care. Adult patients were consented following a urologic procedure, and data was collected prospectively using automated text messaging through post-operative day 28 (August 1, 2019 to February 28, 2021). We measured patient-reported pain intensity, ability to manage pain, and opioid use measured in oxycodone 5 mg tablet equivalents. Outcomes were weighted based on the inverse probability of response to yield representative estimates.
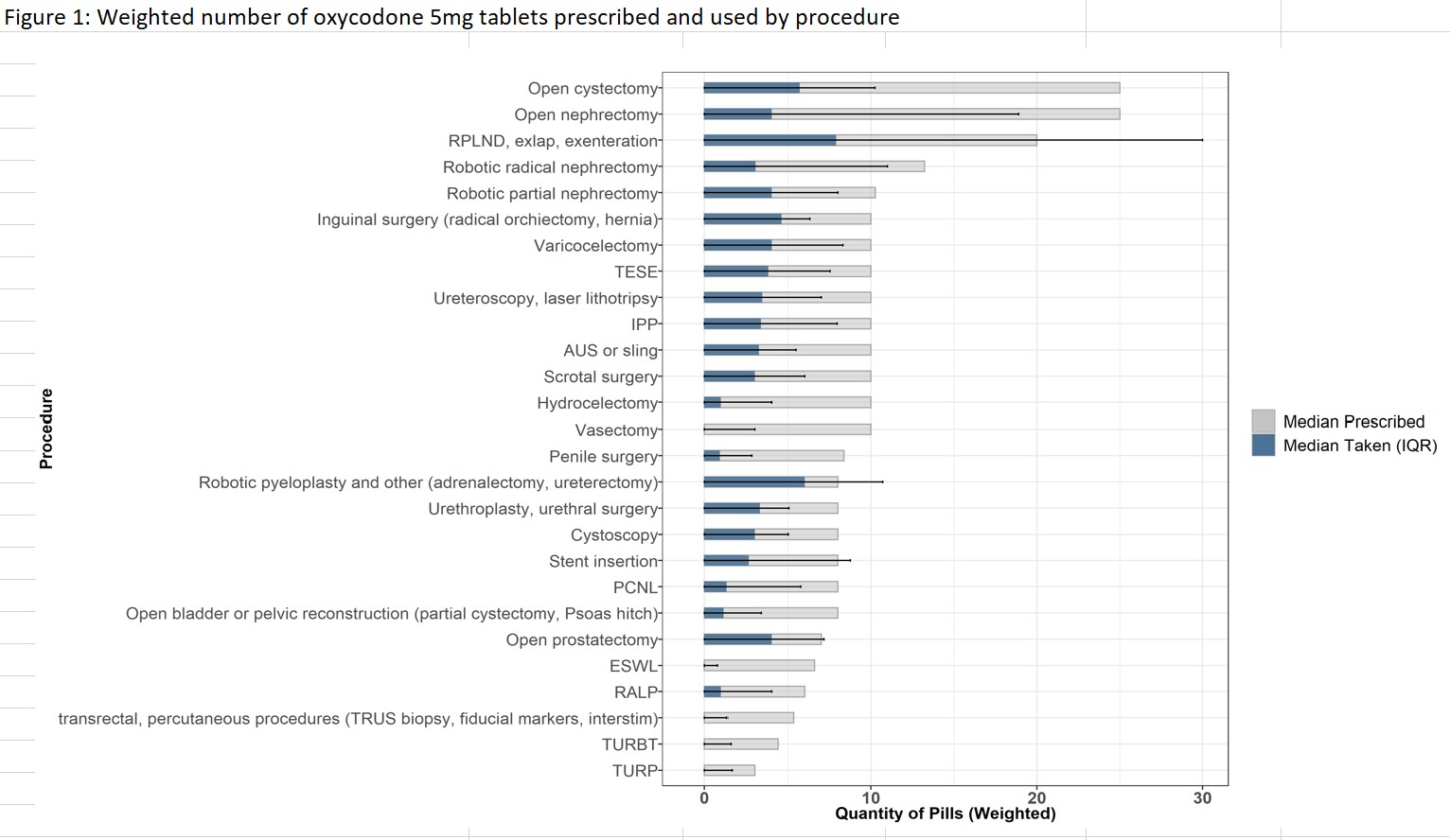
Results: 1015 (%) patients responded to the text-message survey. There was an excess of opioids prescribed relative to postoperative use and pain levels. The median number of pills prescribed was 10 (IQR 6-10), and the median number of pills taken was 2 (IQR 0-6). There was considerable variation in the amount of opioids prescribed across different procedures. By postoperative day 7, the median tablets taken overall was 0. Over the study period, 60.1% (6566) of all tablets prescribed were left unused, and 38.4% of patients did not use any of the prescribed opioids. Across urologic procedures, 6 tablets would accommodate the 75th percentile of patient-reported use, with the exception of major open procedures.
Conclusions: In this prospective study utilizing real-time measurement of opioid use and pain levels with text messaging, there was evidence of dramatic over-prescription of opioids relative to use and pain levels. Patient-reported data, collected via text messaging, can support clinicians and policy leaders in forming national guidelines on evidence-based best practices, personalizing prescriptions and guide shared decision making to decrease opioid excess.
Source of Funding: This project was supported by grant number K12HS026372 (DJL, AKA) from the Agency for Healthcare Research and Quality and HHSF223201810209C from the U.S, Food and Drug Administration (MKD, AKA, ZA), The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality and Food and Drug Administration.
Methods: This was a prospective, observational study investigating patient-reported outcomes and the quantity of opioids prescribed and consumed in the context of care. Adult patients were consented following a urologic procedure, and data was collected prospectively using automated text messaging through post-operative day 28 (August 1, 2019 to February 28, 2021). We measured patient-reported pain intensity, ability to manage pain, and opioid use measured in oxycodone 5 mg tablet equivalents. Outcomes were weighted based on the inverse probability of response to yield representative estimates.
Results: 1015 (%) patients responded to the text-message survey. There was an excess of opioids prescribed relative to postoperative use and pain levels. The median number of pills prescribed was 10 (IQR 6-10), and the median number of pills taken was 2 (IQR 0-6). There was considerable variation in the amount of opioids prescribed across different procedures. By postoperative day 7, the median tablets taken overall was 0. Over the study period, 60.1% (6566) of all tablets prescribed were left unused, and 38.4% of patients did not use any of the prescribed opioids. Across urologic procedures, 6 tablets would accommodate the 75th percentile of patient-reported use, with the exception of major open procedures.
Conclusions: In this prospective study utilizing real-time measurement of opioid use and pain levels with text messaging, there was evidence of dramatic over-prescription of opioids relative to use and pain levels. Patient-reported data, collected via text messaging, can support clinicians and policy leaders in forming national guidelines on evidence-based best practices, personalizing prescriptions and guide shared decision making to decrease opioid excess.
Source of Funding: This project was supported by grant number K12HS026372 (DJL, AKA) from the Agency for Healthcare Research and Quality and HHSF223201810209C from the U.S, Food and Drug Administration (MKD, AKA, ZA), The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality and Food and Drug Administration.


.jpg)
.jpg)