Back
Poster, Podium & Video Sessions
Moderated Poster
MP31: Health Services Research: Quality Improvement & Patient Safety II
MP31-13: Disparities in Race and Ethnicity Reporting in Contemporary Clinical Trials Leading to New Drug Approvals in Urology
Saturday, May 14, 2022
2:45 PM – 4:00 PM
Location: Room 228
Andres Guillen Lozoya*, Vidit Sharma, Bradley Leibovich, Candace Granberg, Patricio Gargollo, Kevin Koo, Rochester, MN
Poster Presenter(s)
Introduction: Population-based clinical trials are the foundation of new drug development and approval. However, trial participants frequently do not represent the racial and ethnic composition of the population they are intended to emulate. To assess the impact of recent efforts to improve trial diversity in urology, this study aimed to assess race and ethnicity reporting in clinical trials (CT) leading to recent urological drug approvals.
Methods: We retrospectively reviewed US Food and Drug Administration new drug approvals for urological indications from 2015 through 2021. Published trials that supported these approvals were analyzed to determine reporting of participant race and ethnicity. We calculated proportional race/ethnicity reporting after censoring trials that did not report race/ethnicity in part or overall. The sample was clustered and analyzed by demographic and clinical variables.
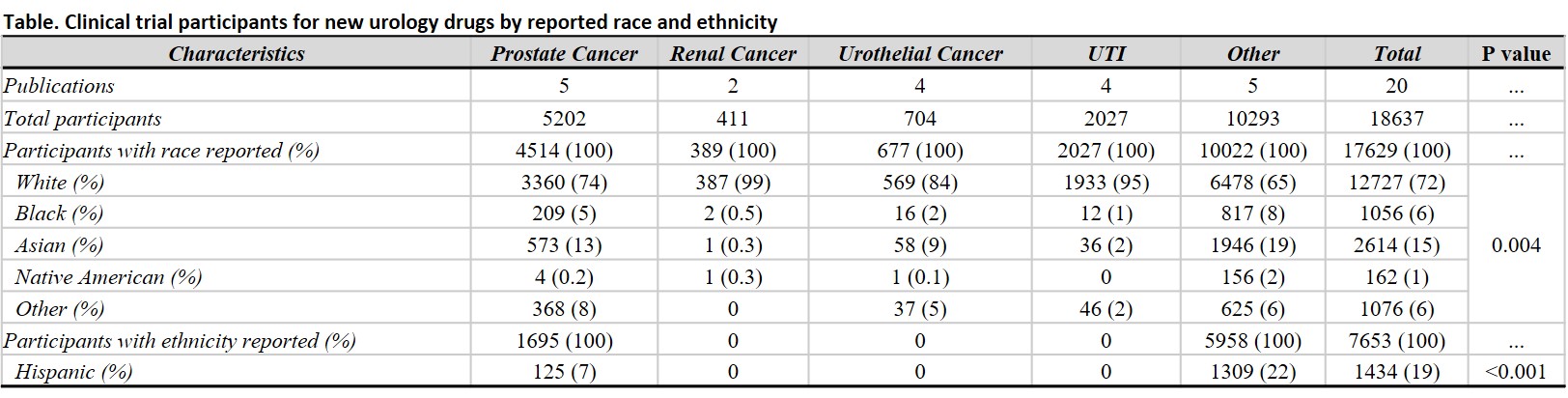
Results: Of 314 new drug trials, a total of 20 were specific for novel therapies in urology with at least 1 race reported as a baseline characteristic. Of the 18,637 trial participants, 17,629 (95%) included race/ethnicity data (Table): 72% White, 15% Asian, 6% Black, 1% Native American, and 6% other unspecified race. Of the total participant sample, only 7,653 (41%) had reported ethnicity; of these, 19% were reported as Hispanic. We found significant differences in reporting rates for participant race and ethnicity rates among trials (p < 0.01). The proportional representation of race/ethnicity among trial participants was compared to US population data from the 2020 Census; White participants were significantly overrepresented in drug trials relative to the overall population (72% vs 58%), while Black participants were significantly underrepresented (6% vs 12%; p<0.001).
Conclusions: Despite efforts to improve the racial and ethnic diversity of clinical trial participants, gaps in race/ethnicity reporting and disparities in representation persist in urological drug trials. Non-White trial participants remain underrepresented relative to the US population for drugs targeting cancer and non-cancer conditions. Renewed efforts are urgently needed to ensure inclusive drug trials in urology.
Source of Funding: None
Methods: We retrospectively reviewed US Food and Drug Administration new drug approvals for urological indications from 2015 through 2021. Published trials that supported these approvals were analyzed to determine reporting of participant race and ethnicity. We calculated proportional race/ethnicity reporting after censoring trials that did not report race/ethnicity in part or overall. The sample was clustered and analyzed by demographic and clinical variables.
Results: Of 314 new drug trials, a total of 20 were specific for novel therapies in urology with at least 1 race reported as a baseline characteristic. Of the 18,637 trial participants, 17,629 (95%) included race/ethnicity data (Table): 72% White, 15% Asian, 6% Black, 1% Native American, and 6% other unspecified race. Of the total participant sample, only 7,653 (41%) had reported ethnicity; of these, 19% were reported as Hispanic. We found significant differences in reporting rates for participant race and ethnicity rates among trials (p < 0.01). The proportional representation of race/ethnicity among trial participants was compared to US population data from the 2020 Census; White participants were significantly overrepresented in drug trials relative to the overall population (72% vs 58%), while Black participants were significantly underrepresented (6% vs 12%; p<0.001).
Conclusions: Despite efforts to improve the racial and ethnic diversity of clinical trial participants, gaps in race/ethnicity reporting and disparities in representation persist in urological drug trials. Non-White trial participants remain underrepresented relative to the US population for drugs targeting cancer and non-cancer conditions. Renewed efforts are urgently needed to ensure inclusive drug trials in urology.
Source of Funding: None


.jpg)
.jpg)
