Back
Poster, Podium & Video Sessions
Moderated Poster
MP32: Surgical Technology & Simulation: Instrumentation & Technology I
MP32-08: Development of a Safe Alternative to Foley Catheter (SafeCath) to Reduce Catheter-Induced Trauma
Saturday, May 14, 2022
2:45 PM – 4:00 PM
Location: Room 225
Hersh Bendre, Graham Lieberman*, Jonathan Kusner, Amy Hao, Francis McGovern, Boston, MA
- GL
Graham M. Lieberman, MD, MBA
Massachusetts General Hospital
Poster Presenter(s)
Introduction: The design of the foley catheter inherently leads to catheter-induced trauma (CIT), which occurs in 1-2% of catheterized patients and results in 200,000 injuries per year. Most major Injuries occur due to intraurethral inflation of the balloon (type 2) or traumatic removal of the inflated balloon (type 3). These lead to increased acute care needs, chronic urethral stricture disease, and increased costs of several billion dollars per year. We describe the development of a novel urinary catheter balloon design which reduces types 2 and 3 CIT.
Methods: We used Systematic Innovative Thinking techniques to develop a urinary catheter design which met the following conditions: 1) Unable to dilate/disrupt the urethra during intraurethral activation, 2) Able to remain internalized when exposed to non-traumatic extraction forces, 3) Able to be removed atraumatically when exposed to extraction forces that induce trauma with a foley catheter. This design was prototyped and tested using ex-vivo porcine bladders.
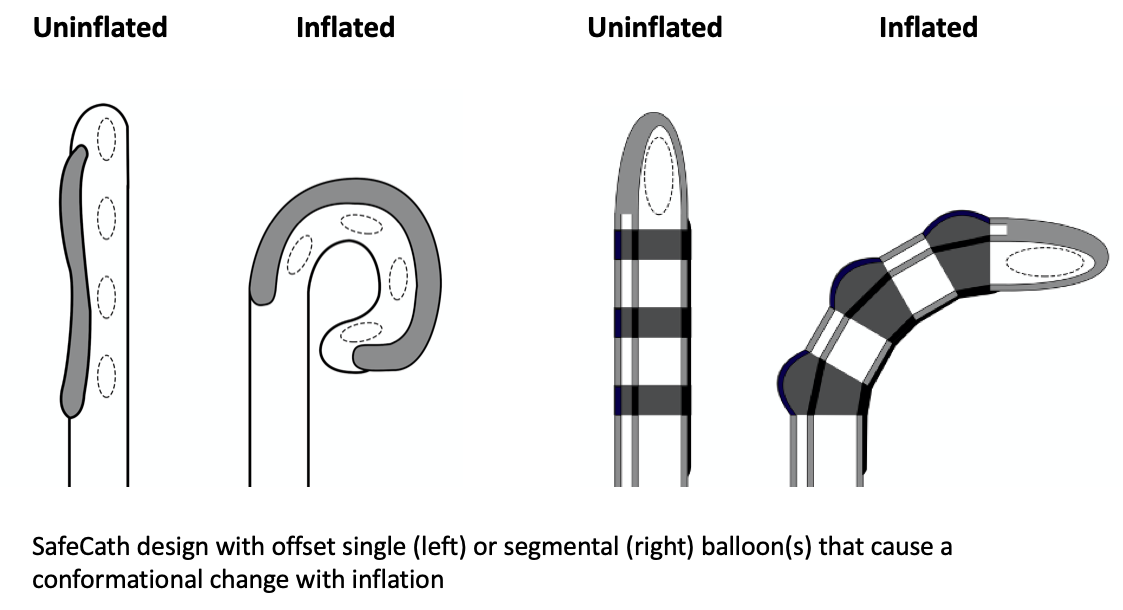
Results: Our design consists of a set of non-radially expanding balloons near the tip of the catheter that lay offset from the longitudinal axis. On inflation, the offset position of the balloons causes differential lengthening of one side of the catheter, resulting in a curling of the catheter tip without radial expansion. Testing demonstrated a maximal diameter of 25 Fr compared to 80 Fr for a standard 10cc foley catheter balloon. SafeCath exerted less than 5 N of sheer force. Mean extraction forces from porcine bladders for the SafeCath and foley catheter were 10.6 N and 27.7 N respectively. Foley catheter extraction resulted in gross urethral disruption, while no trauma was seen with SafeCath.
Conclusions: We designed and tested a urinary catheter with a novel internalization mechanism that reduces the trauma caused by the spherical internalization balloon of foley catheters. Testing demonstrated that SafeCath has a smaller maximal diameter and low sheer force exertion, prohibiting injury with intraurethral inflation. In addition, the SafeCath remains internalized up to a safe threshold extraction force, after which it is atraumatically removed. This preliminary data suggests SafeCath shows promise as a urinary catheter which is able to reduce or eliminate types 2 and 3 CIT.
Source of Funding: None
Methods: We used Systematic Innovative Thinking techniques to develop a urinary catheter design which met the following conditions: 1) Unable to dilate/disrupt the urethra during intraurethral activation, 2) Able to remain internalized when exposed to non-traumatic extraction forces, 3) Able to be removed atraumatically when exposed to extraction forces that induce trauma with a foley catheter. This design was prototyped and tested using ex-vivo porcine bladders.
Results: Our design consists of a set of non-radially expanding balloons near the tip of the catheter that lay offset from the longitudinal axis. On inflation, the offset position of the balloons causes differential lengthening of one side of the catheter, resulting in a curling of the catheter tip without radial expansion. Testing demonstrated a maximal diameter of 25 Fr compared to 80 Fr for a standard 10cc foley catheter balloon. SafeCath exerted less than 5 N of sheer force. Mean extraction forces from porcine bladders for the SafeCath and foley catheter were 10.6 N and 27.7 N respectively. Foley catheter extraction resulted in gross urethral disruption, while no trauma was seen with SafeCath.
Conclusions: We designed and tested a urinary catheter with a novel internalization mechanism that reduces the trauma caused by the spherical internalization balloon of foley catheters. Testing demonstrated that SafeCath has a smaller maximal diameter and low sheer force exertion, prohibiting injury with intraurethral inflation. In addition, the SafeCath remains internalized up to a safe threshold extraction force, after which it is atraumatically removed. This preliminary data suggests SafeCath shows promise as a urinary catheter which is able to reduce or eliminate types 2 and 3 CIT.
Source of Funding: None


.jpg)
.jpg)