Back
Poster, Podium & Video Sessions
Moderated Poster
MP32: Surgical Technology & Simulation: Instrumentation & Technology I
MP32-17: Effectivity of a Novel Implantable Device (Stimrouter) for the Treatment of Overactive Bladder Syndrome: 30 Months Results of a Multi-Centre Study
Saturday, May 14, 2022
2:45 PM – 4:00 PM
Location: Room 225
Mohammad Khaneshi*, Heerlen, Netherlands, Mohammad Sajjad Rahnama'i, Aachen, Germany, Sakineh Haj Ebrahimi, Tabriz, Islamic Republic of Iran
- MK
Poster Presenter(s)
Introduction: After failing conservative treatment alternatives for these patients such as behavioral modification and pharmaceutical management, intravesical Botulinum toxin injection, sacral neuromodulation (SNM) and percutaneous tibial nerve stimulation (PTNS) are well-established third-line treatment options. SNM and PTNS, have not seen any significant improvement of the devices over the last decade or since FDA approval in 1997. In this study a new, battery-free implantable tined lead device (StimRouter™ by Bioness) in a multi-center study is evaluated.
Methods: From May 2019, 7 consecutive patients with urgency incontinence and detrusor over activity underwent a procedure under local anesthesia in which a battery-free tined lead electrode was implanted on the medial side of the ankle (Figure 1). This lead was implanted by the same surgeon in all cases through a single 5 mm incision and after the appropriate response of electrical stimulation, (flexion of the first toe and paresthesia of the foot) was looked. After identifying correct position, the lead was inserted through Seldinger technique and after a final electrical check of the appropriate responses, subcutaneously tunneled for 10 cm in the proximal direction of the medial side of the ankle. The total procedure time was 15-25 minutes.Patients were advised to stimulate their tibial nerve with a hand-held remote at home for an hour per day.
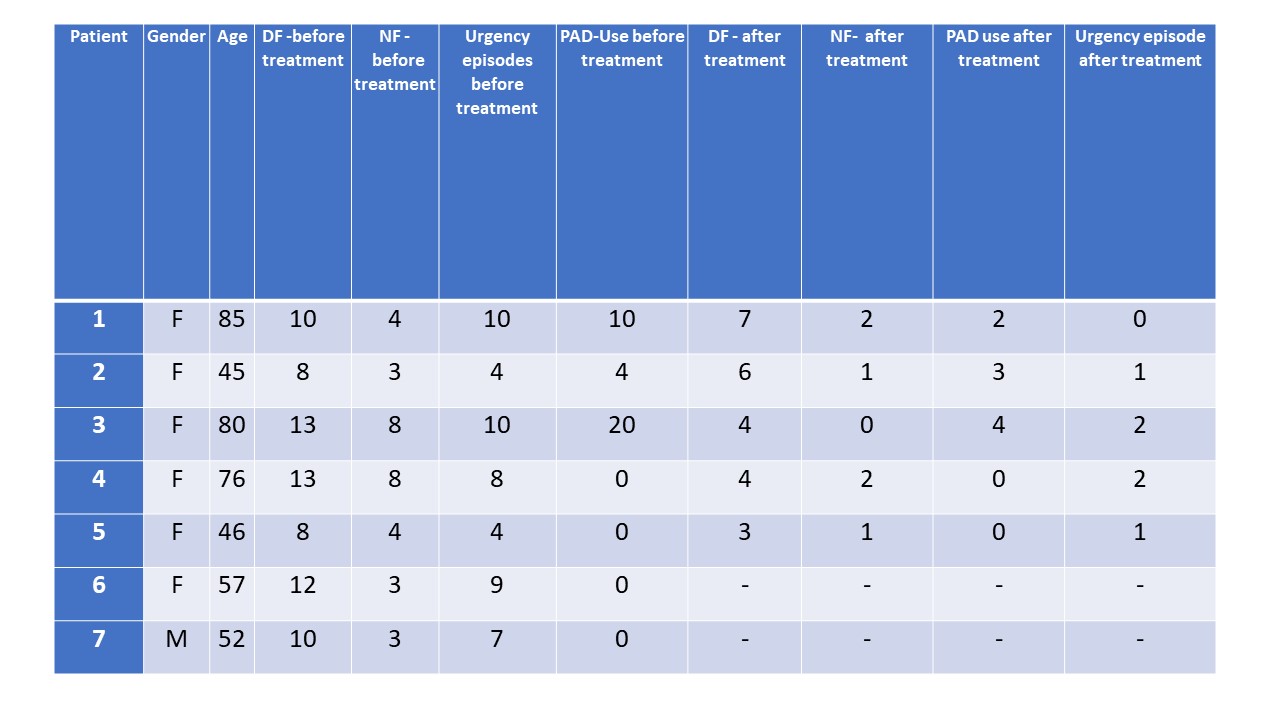
Results: All 7 patients reported an improvement in the urgency and incontinence episodes and a significant reduction of incontinence-pad use after treatment. In addition, both day-time as well as night-time frequency was reduced in all implanted patients. (Figure1)
Conclusions: Our multicenter data presented here are the first follow up data on feasibility and effect of the battery-free implantable tibial nerve stimulation device (Stimrouter) for the treatment of urinary incontinence. There were no adverse events and all implanted patients reported a significant improvement in urgency and incontinence episodes as well as pad use and day-time
and night-time frequency. We can conclude that tibial nerve stimulation with Stimrouter could be a very promising therapy for patients with refractory idiopathic OAB
Source of Funding: None
Methods: From May 2019, 7 consecutive patients with urgency incontinence and detrusor over activity underwent a procedure under local anesthesia in which a battery-free tined lead electrode was implanted on the medial side of the ankle (Figure 1). This lead was implanted by the same surgeon in all cases through a single 5 mm incision and after the appropriate response of electrical stimulation, (flexion of the first toe and paresthesia of the foot) was looked. After identifying correct position, the lead was inserted through Seldinger technique and after a final electrical check of the appropriate responses, subcutaneously tunneled for 10 cm in the proximal direction of the medial side of the ankle. The total procedure time was 15-25 minutes.Patients were advised to stimulate their tibial nerve with a hand-held remote at home for an hour per day.
Results: All 7 patients reported an improvement in the urgency and incontinence episodes and a significant reduction of incontinence-pad use after treatment. In addition, both day-time as well as night-time frequency was reduced in all implanted patients. (Figure1)
Conclusions: Our multicenter data presented here are the first follow up data on feasibility and effect of the battery-free implantable tibial nerve stimulation device (Stimrouter) for the treatment of urinary incontinence. There were no adverse events and all implanted patients reported a significant improvement in urgency and incontinence episodes as well as pad use and day-time
and night-time frequency. We can conclude that tibial nerve stimulation with Stimrouter could be a very promising therapy for patients with refractory idiopathic OAB
Source of Funding: None


.jpg)
.jpg)