Back
Poster, Podium & Video Sessions
Moderated Poster
MP34: Infertility: Therapy
MP34-05: Impact of Nasal, Intramuscular, and Subcutaneous Testosterone pellets on Intratesticular Testosterone: Evaluation of Data from Two Randomized Clinical Trials
Saturday, May 14, 2022
4:30 PM – 5:45 PM
Location: Room 225
Parris Diaz*, Rohit Reddy, Ruben Blachman-Braun, Alexandra Dullea, Isaac J. Zucker, Daniel C. Gonzalez, Eliyahu Kresch, Ranjith Ramasamy, Miami, FL
.jpg)
Parris Antonio Diaz, BS
University of Miami/DGSOM at UCLA
Poster Presenter(s)
Introduction: Testosterone replacement therapy (TRT) can potentially cause decreased spermatogenesis and subsequent infertility due to decrease in intratesticular testosterone (ITT) production. Serum 17-hydroxyprogesterone (17-OHP) is a reliable surrogate for ITT that is critical for preserving male fertility. We hypothesized that long-acting T formulations will decrease ITT compared to short-acting formulations. We evaluated data from two open-label, randomized, two-arm clinical trials amongst different testosterone therapies with subcutaneous T pellets (TP), Intranasal Testosterone (NT) or Intramuscular Testosterone cypionate (TC).
Methods: A total of 75 symptomatic hypogonadal men (2 serum T < 300 ng/dL drawn before 10AM) were randomized into open-label randomized clinical trials. Eligible subjects received 800mg TP, 11mg TID NT or 200mg x 2 weeks TC. Lab values were evaluated at baseline and follow-up. The primary outcome was changes in serum 17-OHP. Secondary outcome was changes in serum T. Data analyzed via Kruskal Wallis and Chi square test.
Trials: NCT04439799 (June 19,2020) & NCT04523480 (August 21,2020).
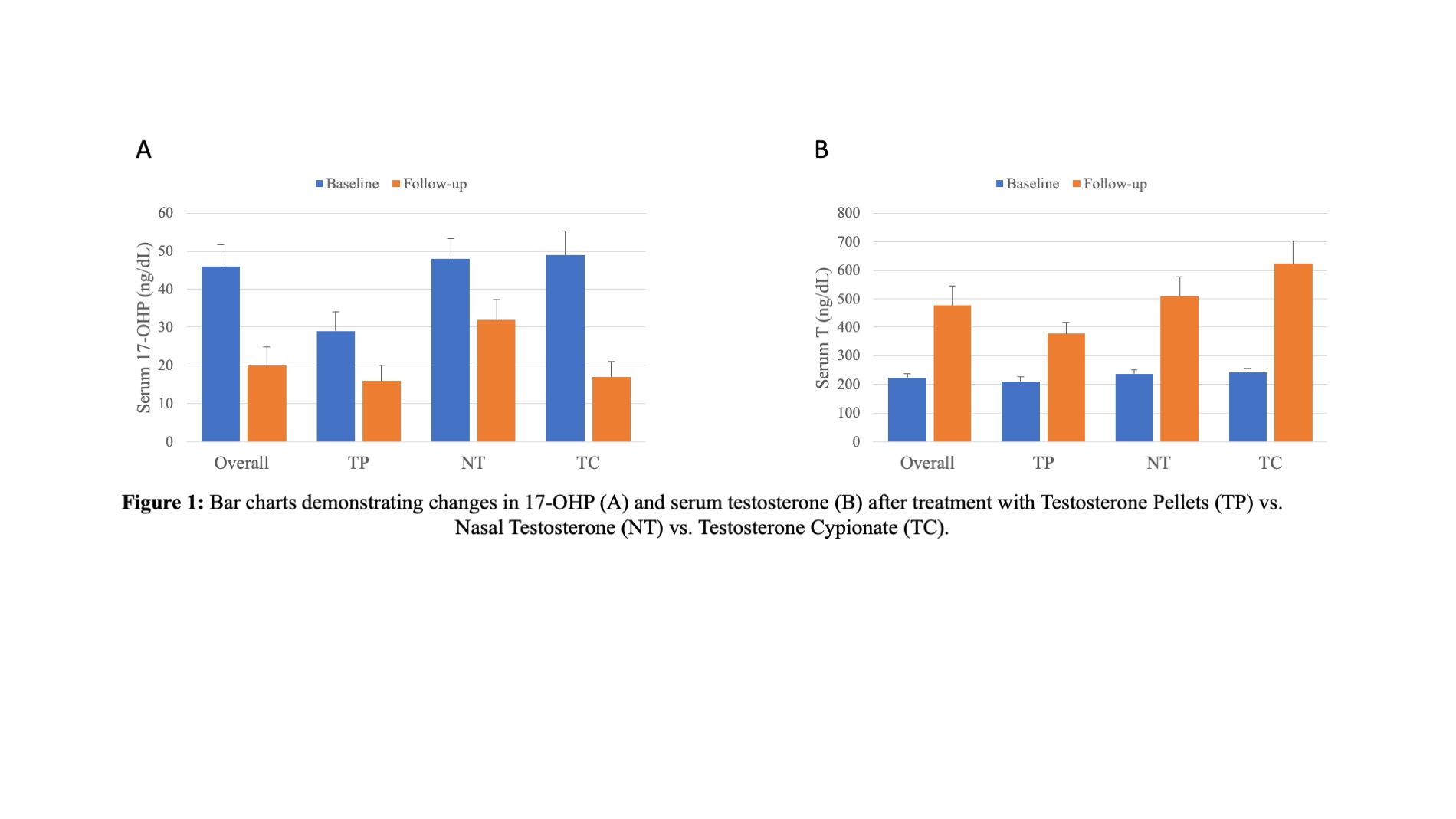
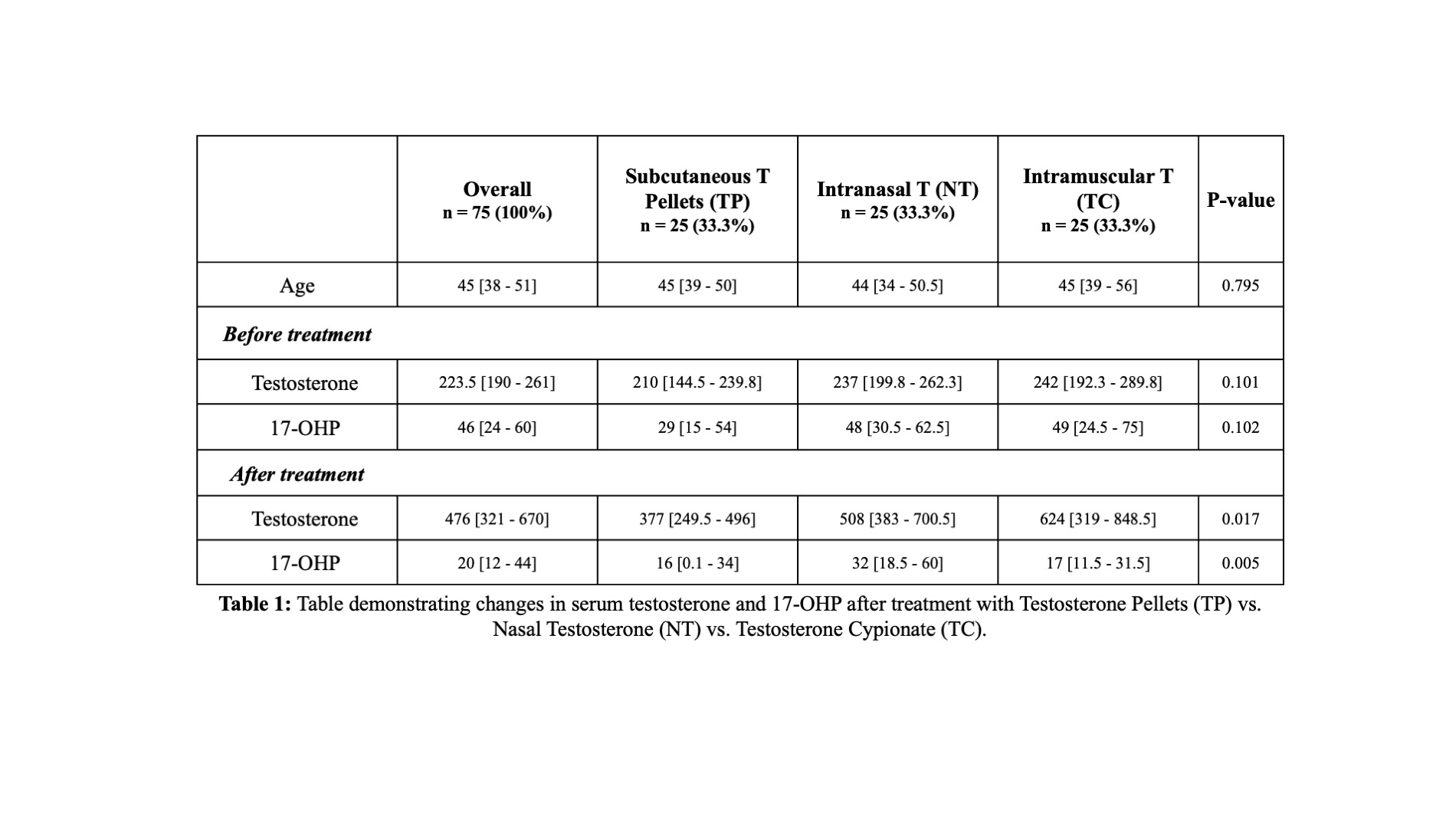
Results: Median participant age was 45 years old, with overall baseline 17-OHP of 46 and serum testosterone of 223.5 ng/dL. Serum 17-OHP significantly decreased in all three TRT regimens (Table 1). The 4-month change in 17-OHP in the NT group (-33.3% from baseline) was less than the change seen in TC (-65.3% from baseline) or TP (-44% from baseline) (p=0.005) . All T formulations increased serum testosterone levels at follow-up, with the largest increase seen in TC (+157.6%), followed by NT (+114.3%), and TP (+79.6%) (p=0.005).
Conclusions: Short-acting nasal testosterone appears to decrease 17-OHP less than long-acting T formulations. All modalities saw significant increases in serum T levels at follow-up. Intranasal T and other short acting T formulations may better preserve ITT and be beneficial for hypogonadal men seeking to maintain fertility potential while on TRT.
Source of Funding: Investigator Initiated Grant funded by Acerus Pharmaceuticals and Empower Pharmacy (RR). This work was also supported by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to RR.
Methods: A total of 75 symptomatic hypogonadal men (2 serum T < 300 ng/dL drawn before 10AM) were randomized into open-label randomized clinical trials. Eligible subjects received 800mg TP, 11mg TID NT or 200mg x 2 weeks TC. Lab values were evaluated at baseline and follow-up. The primary outcome was changes in serum 17-OHP. Secondary outcome was changes in serum T. Data analyzed via Kruskal Wallis and Chi square test.
Trials: NCT04439799 (June 19,2020) & NCT04523480 (August 21,2020).
Results: Median participant age was 45 years old, with overall baseline 17-OHP of 46 and serum testosterone of 223.5 ng/dL. Serum 17-OHP significantly decreased in all three TRT regimens (Table 1). The 4-month change in 17-OHP in the NT group (-33.3% from baseline) was less than the change seen in TC (-65.3% from baseline) or TP (-44% from baseline) (p=0.005) . All T formulations increased serum testosterone levels at follow-up, with the largest increase seen in TC (+157.6%), followed by NT (+114.3%), and TP (+79.6%) (p=0.005).
Conclusions: Short-acting nasal testosterone appears to decrease 17-OHP less than long-acting T formulations. All modalities saw significant increases in serum T levels at follow-up. Intranasal T and other short acting T formulations may better preserve ITT and be beneficial for hypogonadal men seeking to maintain fertility potential while on TRT.
Source of Funding: Investigator Initiated Grant funded by Acerus Pharmaceuticals and Empower Pharmacy (RR). This work was also supported by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society to RR.



.jpg)
.jpg)