Back
Poster, Podium & Video Sessions
Moderated Poster
MP35: Sexual Function/Dysfunction: Medical, Hormonal & Non-surgical Therapy I
MP35-09: Linear Relationship between the Time of Dosing and 24-Hour Average Concentration of Total Testosterone in Men Treated with an Oral Testosterone Undecanoate Capsule (JATENZO&[reg])
Saturday, May 14, 2022
4:30 PM – 5:45 PM
Location: Room 222
Ronald Swerdloff, Torrance, CA, Jason Kovac*, Indianapolis, IN, Christina Wang, Torrance, CA, Marc Gittelman, Aventura, FL, B. Woun Seo, Jay Newmark, Northbrook, IL, Nestor Rohowsky, LaGrange, IL, Robert Dudley, Northbrook, IL
- JK
Jason R. Kovac, MD, PHD, FACS, FRCSC
Director, Men's Health Center
Men's Health Center
Poster Presenter(s)
Introduction: In 2019, a novel, first-in-class testosterone (T) replacement therapy (TRT), oral testosterone undecanoate (TU) was approved by the US FDA for the treatment of male hypogonadism. Concordance studies have shown that total T at 6 hrs after the morning TU dose best corresponds to the total T 24-hr average concentration (Cavg). Hence, product labeling directs clinicians to measure serum T 6 hrs after a morning TU dose. However, testing at 6 hrs may pose scheduling difficulties for some men. Therefore, the relationship between other T assay time points and Cavg was examined to see if a conversion factor could be derived to convert T values collected at earlier or later times than directed in product labeling (i.e., 6 hrs) into values that reflect true Cavg.
Methods: Hypogonadal men, age 18 – 65 y/o, were recruited into a randomized, open-label, multicenter, dose-titration trial. Dose titration was based on Cavg calculated from serial pharmacokinetic (PK) samples. Patients had two dose adjustments based Cavg. Because of the need for titration, there were three different PK visits. Ratio between the different timepoints and Cavg were determined for the oral TU PK samples following morning drug administration.
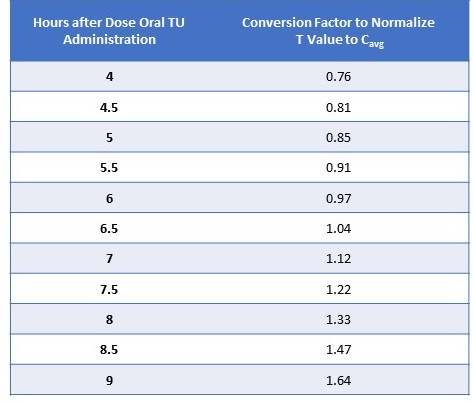
Results: With the values from all three PK days pooled, there was a linear relationship between T concentrations at 4, 6, and 9 hrs and Cavg (r2= 0.35). The r2 for the three visits were similar: 1st PK visit, r2= 0.33; 2nd PK visit, r2= 0.36; final PK visit, r2= 0.35. A factor based on the following equation was derived to enable conversion of T values assessed at a times other than 6 hrs (i.e., time of blood draw) into a close approximation of Cavg: (1.870 – 0.14) x (hours after AM dose). Table summarizes the conversion factors to normalize T to Cavg at various T assay time points. To illustrate, for a serum T value drawn at either 4 or 8 hrs after the morning oral TU dose, the normalized (or corrected) Cavg concentration would be approximately 75% or 133% of the drawn value, respectively.
Conclusions: While dose titration for this novel oral TRT is based on a serum T level drawn at 6 hours after the AM dose, the T level can be estimated from samples obtained at other timepoints using a conversion factor.
Source of Funding: Clarus Therapeutics
Methods: Hypogonadal men, age 18 – 65 y/o, were recruited into a randomized, open-label, multicenter, dose-titration trial. Dose titration was based on Cavg calculated from serial pharmacokinetic (PK) samples. Patients had two dose adjustments based Cavg. Because of the need for titration, there were three different PK visits. Ratio between the different timepoints and Cavg were determined for the oral TU PK samples following morning drug administration.
Results: With the values from all three PK days pooled, there was a linear relationship between T concentrations at 4, 6, and 9 hrs and Cavg (r2= 0.35). The r2 for the three visits were similar: 1st PK visit, r2= 0.33; 2nd PK visit, r2= 0.36; final PK visit, r2= 0.35. A factor based on the following equation was derived to enable conversion of T values assessed at a times other than 6 hrs (i.e., time of blood draw) into a close approximation of Cavg: (1.870 – 0.14) x (hours after AM dose). Table summarizes the conversion factors to normalize T to Cavg at various T assay time points. To illustrate, for a serum T value drawn at either 4 or 8 hrs after the morning oral TU dose, the normalized (or corrected) Cavg concentration would be approximately 75% or 133% of the drawn value, respectively.
Conclusions: While dose titration for this novel oral TRT is based on a serum T level drawn at 6 hours after the AM dose, the T level can be estimated from samples obtained at other timepoints using a conversion factor.
Source of Funding: Clarus Therapeutics


.jpg)
.jpg)