Back
Poster, Podium & Video Sessions
Best Poster Award
MP36: Renal Transplantation & Vascular Surgery
MP36-05: A novel method of CD31 combined ABO carbohydrate antigen microarray predicts acute antibody mediated rejection in ABO-incompatible kidney transplantation
Sunday, May 15, 2022
7:00 AM – 8:15 AM
Location: Room 228
Masayuki Tasaki*, Niigata, Japan, Hiroaki Tateno, Takashi Sato, Azusa Tomioka, Hiroyuki Kaji, Hisashi Narimatsu, Tsukuba, Japan, Kazuhide Saito, Toshinari Aoki, Masami Kamimura, Takashi Ushiki, Yutaka Yoshida, Niigata, Japan, Kota Takahashi, Tokyo, Japan, Yoshihiko Tomita, Niigata, Japan

Masayuki Tasaki, MD,Phd
Graduate School of Medical and Dental Sciences, Niigata University
Poster Presenter(s)
Introduction: Isohemagglutinin assays employing red blood cells (RBCs) are the most common assays used to measure antibody titer in ABO-incompatible kidney transplantation (ABOi KTx). However, ABO blood group antigens expressed on RBCs are not identical to those of the kidney due to different proteins linked to ABO carbohydrate antigens. Antibody titers measured by isohemagglutinin assays do not always correlate with clinical outcome and a new method is necessary to precisely predict acute antibody mediated rejection (ABMR) following ABOi KTx.
Methods: We previously reported that CD31 was the most abundant protein linked to ABO carbohydrate antigens on kidney endothelial cells, and developed a new method to measure antibody titer using a microarray of recombinant CD31 (rCD31) linked to ABO carbohydrate antigens (CD31-ABO microarray. Figure 1). To confirm clinical use, a total of 252 plasma samples including volunteers, hemodialysis patients and transplant recipients were examined by both of CD31-ABO microarray and isohemagglutinin assays.
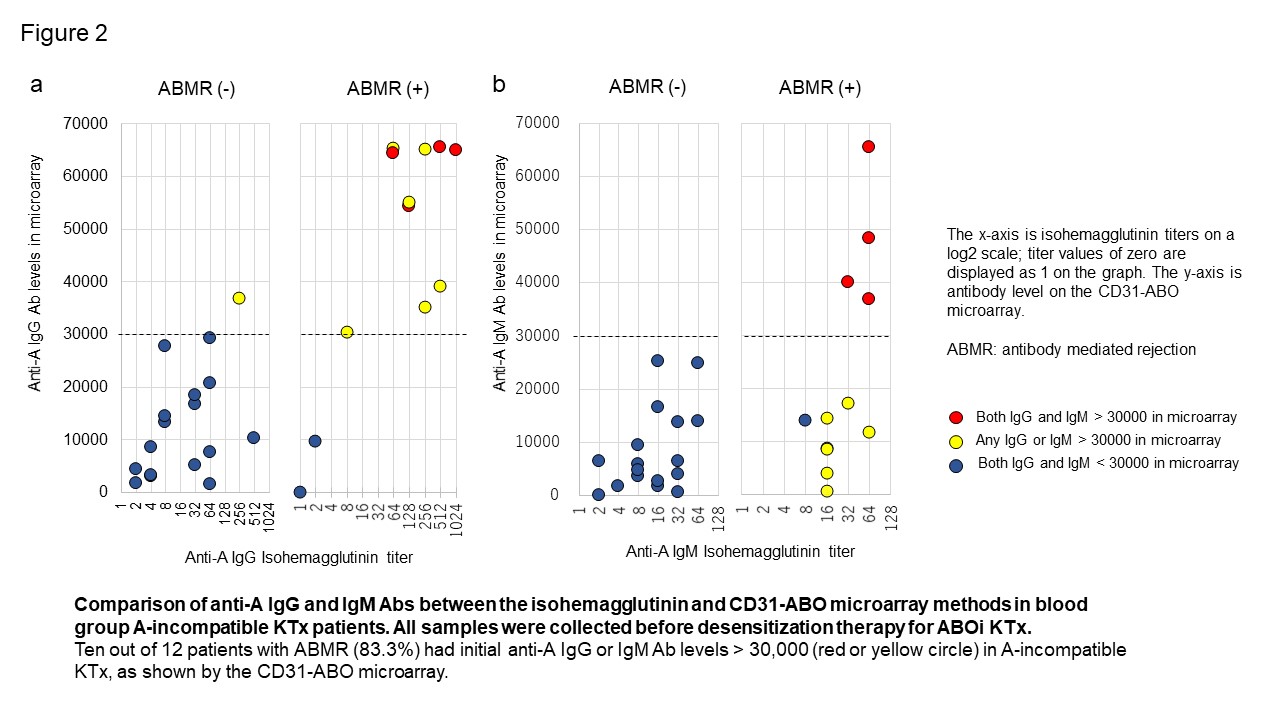
Results: Mass spectrometry analysis suggested that rCD31 and native CD31 had similar ABO glycan, meaning CD31-ABO microarray were the mimic of ABO antigens on renal endothelial cells. In transplant recipients, any initial IgG or IgM antibody intensity > 30,000 against the donor blood type in the CD31-ABO microarray showed higher sensitivity, specificity, positive predictive value, and negative predictive value to predict acute ABMR following ABOi KTx, compared to isohemagglutinin assays. Representative data were shown in Figure 2 (Blood group A-incompatible). Ten out of 12 patients with ABMR (83.3%) had anti-A IgG Ab levels > 30,000 in the CD31-ABO microarray, in contrast, only 1 out of 17 patients without ABMR (5.9%) had anti-A IgG Ab levels > 30,000 (Figure 5a). No one had anti-A IgM Ab levels > 30,000 in the ABMR (-) group (Figure 5b).
Conclusions: Use of a CD31-ABO microarray will contribute to precisely predicting ABMR following ABOi KTx in advance.
Source of Funding: This study was supported by JSPS KAKENHI Grant Number 17K11195.
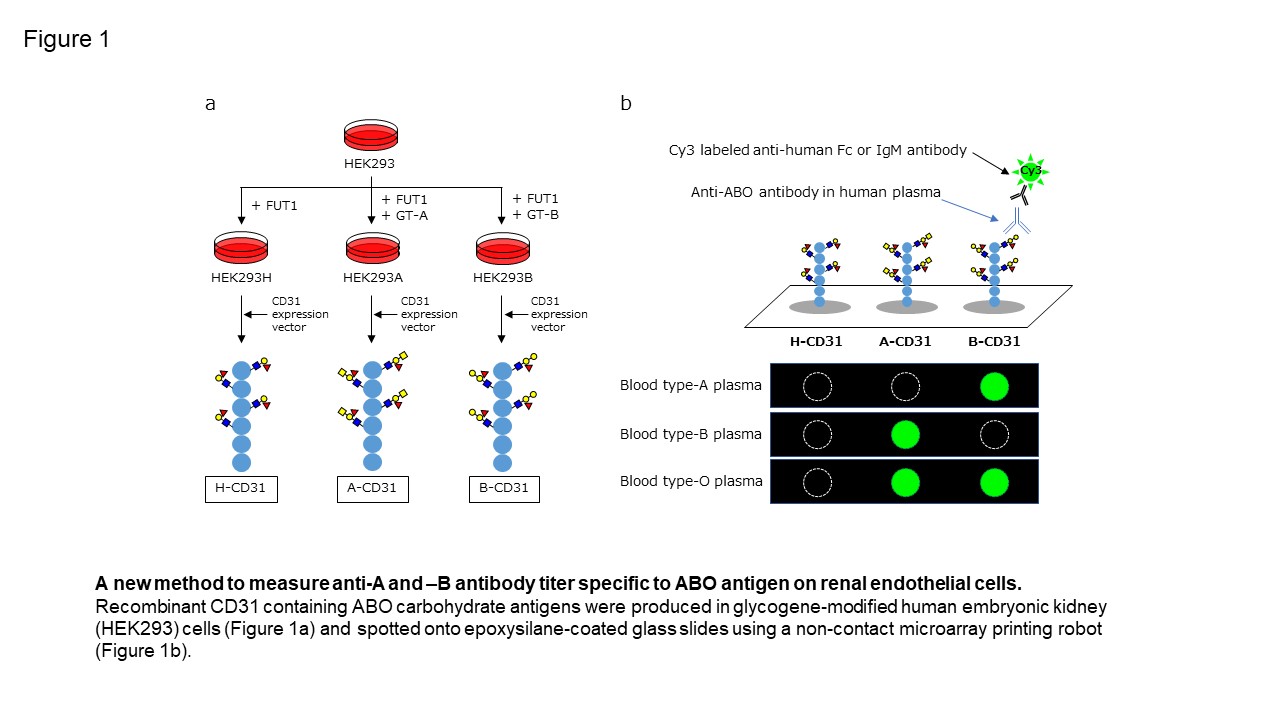
Methods: We previously reported that CD31 was the most abundant protein linked to ABO carbohydrate antigens on kidney endothelial cells, and developed a new method to measure antibody titer using a microarray of recombinant CD31 (rCD31) linked to ABO carbohydrate antigens (CD31-ABO microarray. Figure 1). To confirm clinical use, a total of 252 plasma samples including volunteers, hemodialysis patients and transplant recipients were examined by both of CD31-ABO microarray and isohemagglutinin assays.
Results: Mass spectrometry analysis suggested that rCD31 and native CD31 had similar ABO glycan, meaning CD31-ABO microarray were the mimic of ABO antigens on renal endothelial cells. In transplant recipients, any initial IgG or IgM antibody intensity > 30,000 against the donor blood type in the CD31-ABO microarray showed higher sensitivity, specificity, positive predictive value, and negative predictive value to predict acute ABMR following ABOi KTx, compared to isohemagglutinin assays. Representative data were shown in Figure 2 (Blood group A-incompatible). Ten out of 12 patients with ABMR (83.3%) had anti-A IgG Ab levels > 30,000 in the CD31-ABO microarray, in contrast, only 1 out of 17 patients without ABMR (5.9%) had anti-A IgG Ab levels > 30,000 (Figure 5a). No one had anti-A IgM Ab levels > 30,000 in the ABMR (-) group (Figure 5b).
Conclusions: Use of a CD31-ABO microarray will contribute to precisely predicting ABMR following ABOi KTx in advance.
Source of Funding: This study was supported by JSPS KAKENHI Grant Number 17K11195.

.jpg)
.jpg)