Back
Poster, Podium & Video Sessions
Moderated Poster
MP36: Renal Transplantation & Vascular Surgery
MP36-11: Induction of Immune Tolerance after Haploidentical Living Donor Kidney Transplant
Sunday, May 15, 2022
7:00 AM – 8:15 AM
Location: Room 228
Nima Nassiri*, Erik Lum, Monica Mead, Gabriel Danovitch, H. Albin Gritsch, Neil Kogut, Jeffrey Veale, Los Angeles, CA
- NN
Nima Nassiri, MD
Fellow
UCLA
Poster Presenter(s)
Introduction: Though kidney transplants improve survival and quality of life for patients with end-stage kidney disease, the need for lifelong immunosuppression limits allograft lifespan and predisposes patients to a multitude of infectious and malignant complications. Herein, we propose a novel protocol to allow withdrawal from immunosuppressive drugs while maintaining normal graft function.
Methods: In this single-center, feasibility study, appropriately selected dual haploidentical sibling donor and recipient pairs participate in combined living donor kidney and hematopoietic stem cell (HSC) transplantation with the goal of inducing transplant tolerance. Immediately after kidney transplantation, recipients undergo a conditioning regimen of total lymphoid irradiation (TLI) and rabbit antithymocyte globulin (rATG) followed by an intravenous infusion of mobilized peripheral blood CD34+ cells (= 5x106 cells/kg) and CD3+ cells (5x106 cells/kg) from an HLA-identical sibling living donor (Figure 1).
Results: Two donor/recipient pairs thus far have been enrolled and followed for six and eight months. Both recipients demonstrate sustained chimerism of 38% and 7% (goal > 5%). Both patients have discontinued prednisone and mycophenolate mofetil and are on a single agent tacrolimus on a tapering schedule, with anticipation of complete immunosuppression withdrawal at 12 months post-transplant. There have been no episodes of allograft rejection or failure and no evidence of graft vs host disease. Allograft function remains excellent in both recipients, with stable serum creatinine values of 0.86 and 1.28 mg/dL. There were zero grade III or higher adverse events in both donor and recipients.
Conclusions: Thus far, the UCLA transplant tolerance protocol for dual haploidentical living kidney transplantation has demonstrated sustained chimerism and excellent allograft function on minimal immunosuppression en route to total immunosuppression withdrawal. Continued enrollment and follow up will demonstrate the durability of these outcomes.
Source of Funding: OneLegacy Foundation
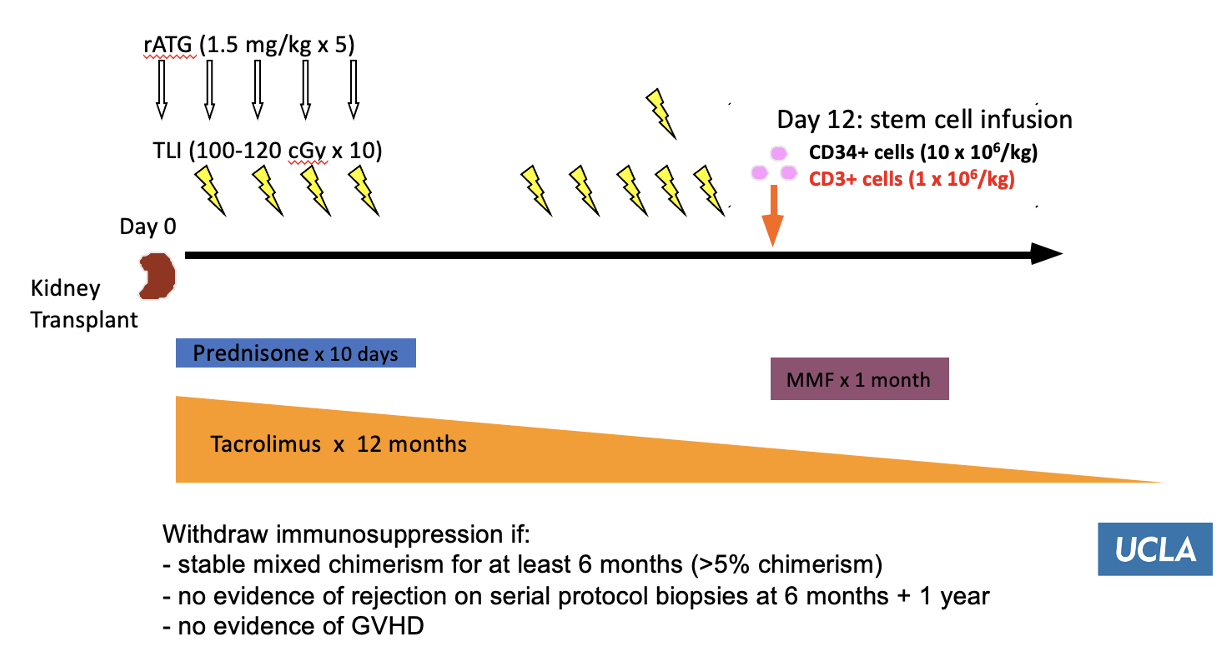
Methods: In this single-center, feasibility study, appropriately selected dual haploidentical sibling donor and recipient pairs participate in combined living donor kidney and hematopoietic stem cell (HSC) transplantation with the goal of inducing transplant tolerance. Immediately after kidney transplantation, recipients undergo a conditioning regimen of total lymphoid irradiation (TLI) and rabbit antithymocyte globulin (rATG) followed by an intravenous infusion of mobilized peripheral blood CD34+ cells (= 5x106 cells/kg) and CD3+ cells (5x106 cells/kg) from an HLA-identical sibling living donor (Figure 1).
Results: Two donor/recipient pairs thus far have been enrolled and followed for six and eight months. Both recipients demonstrate sustained chimerism of 38% and 7% (goal > 5%). Both patients have discontinued prednisone and mycophenolate mofetil and are on a single agent tacrolimus on a tapering schedule, with anticipation of complete immunosuppression withdrawal at 12 months post-transplant. There have been no episodes of allograft rejection or failure and no evidence of graft vs host disease. Allograft function remains excellent in both recipients, with stable serum creatinine values of 0.86 and 1.28 mg/dL. There were zero grade III or higher adverse events in both donor and recipients.
Conclusions: Thus far, the UCLA transplant tolerance protocol for dual haploidentical living kidney transplantation has demonstrated sustained chimerism and excellent allograft function on minimal immunosuppression en route to total immunosuppression withdrawal. Continued enrollment and follow up will demonstrate the durability of these outcomes.
Source of Funding: OneLegacy Foundation


.jpg)
.jpg)