Back
Poster, Podium & Video Sessions
Moderated Poster
MP37: Infections/Inflammation/Cystic Disease of the Genitourinary Tract: Prostate & Genitalia
MP37-05: A systematic review and meta-regression of placebo effect in chronic prostatitis/chronic pelvic pain syndrome
Sunday, May 15, 2022
7:00 AM – 8:15 AM
Location: Room 225
Andrey Morozov*, Andrey Bazarkin, Diana Babaevskaya, Mark Taratkin, Vasiliy Kozlov, Aleksandr Suvorov, Leonid Spivak, Jonathan Mcfarland, Moscow, Russian Federation, Giorgio Russo, Catania, Italy, Dmitry Enikeev, Moscow, Russian Federation
- AM
Poster Presenter(s)
Introduction: The purpose of this study was to evaluate the placebo effect on symptoms of CP/CPPS in order to improve future clinical trials.
Methods: We conducted a search in 3 databases (Scopus, MEDLINE and Web of Science) on the query "placebo AND (prostatitis OR CPP OR "pelvic pain")" to identify double-blind placebo-controlled clinical trials on treatment of CP/CPPS published before April 2021 (Prospero CRD42021252403).
The scope of the review according to the PICOS framework is as follows: P - Patients suffering from chronic prostatitis/chronic pelvic pain syndrome; I – Placebo; C – Symptoms before and after treatment; O – NIH-CPSI score, IPSS score, Qmax, PVR; S – Randomized controlled trials. The primary outcome was National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) score. Secondary outcomes included Qmax, PVR, IPSS and prostate volume.
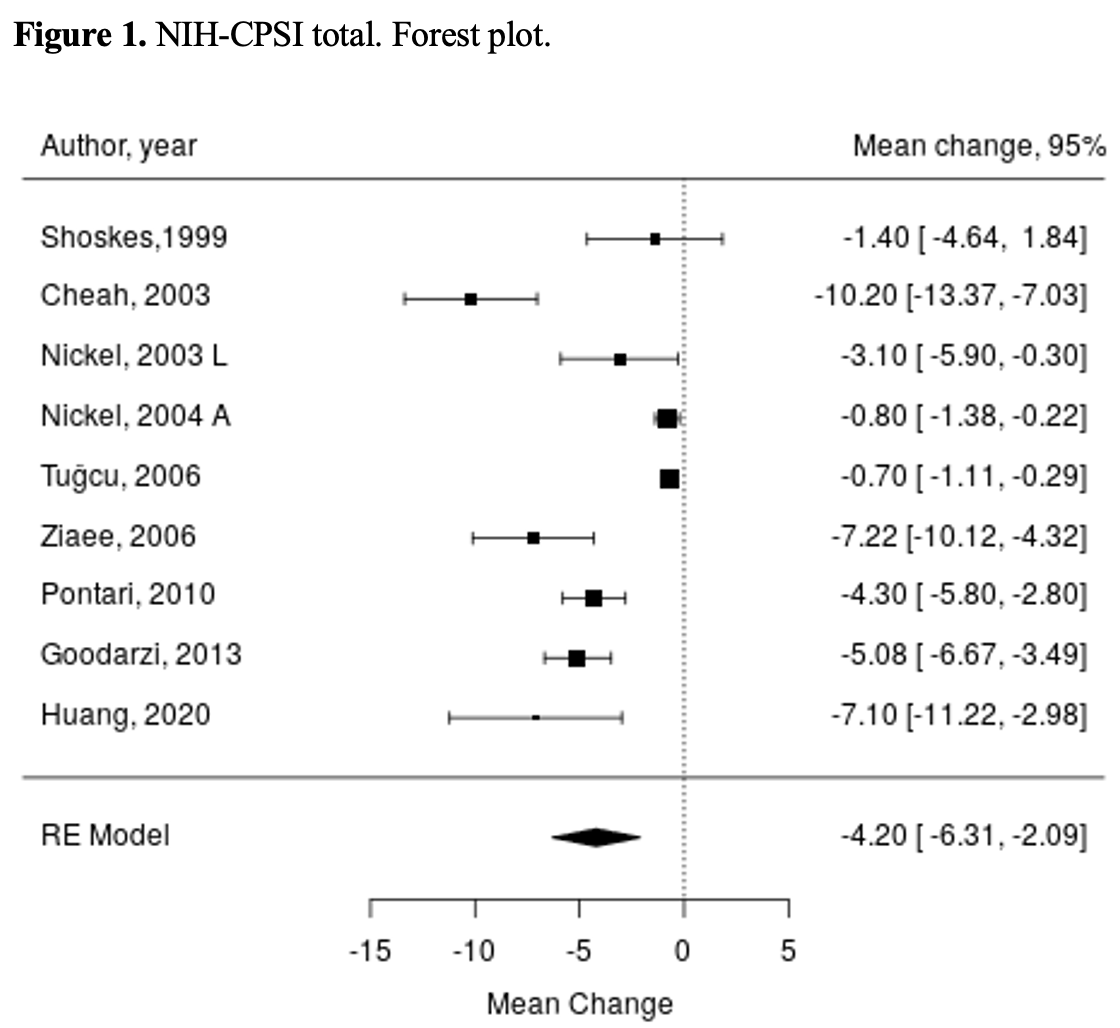
Results: A total of 3502 studies were identified. Placebo arms of 42 articles (5512 patients, median 31 patients) were included in the systematic review. Systematic review identified positive changes in the primary endpoint, meta-analysis of 10 articles found that NIH-CPSI total score results were significantly influenced by placebo, mean difference -4.2 (95% CI -6.31, -2.09), Figure 1. Mean difference of NIH-CPSI pain domain was -2.31 (95% CI -3.4, -1.21), urinary domain -1.12 (95% CI -1.62, -0.62), quality of life domain -1.67 (95% CI -2.38, -0.96); p<0.001 for all. Qmax mean change from baseline was 0.68 (95% CI -0.85, 2.22, p=0.38). Systematic review showed no significant changes in pain, measured by VAS or other scores, IPSS and PVR.
Conclusions: Placebo significantly affected the subjective parameters (NIH-CPSI) and had a minor effect on different measurements of pain, whereas there was no long-term effect on IPSS and the objective measurements (Qmax, PVR).
Source of Funding: None
Methods: We conducted a search in 3 databases (Scopus, MEDLINE and Web of Science) on the query "placebo AND (prostatitis OR CPP OR "pelvic pain")" to identify double-blind placebo-controlled clinical trials on treatment of CP/CPPS published before April 2021 (Prospero CRD42021252403).
The scope of the review according to the PICOS framework is as follows: P - Patients suffering from chronic prostatitis/chronic pelvic pain syndrome; I – Placebo; C – Symptoms before and after treatment; O – NIH-CPSI score, IPSS score, Qmax, PVR; S – Randomized controlled trials. The primary outcome was National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) score. Secondary outcomes included Qmax, PVR, IPSS and prostate volume.
Results: A total of 3502 studies were identified. Placebo arms of 42 articles (5512 patients, median 31 patients) were included in the systematic review. Systematic review identified positive changes in the primary endpoint, meta-analysis of 10 articles found that NIH-CPSI total score results were significantly influenced by placebo, mean difference -4.2 (95% CI -6.31, -2.09), Figure 1. Mean difference of NIH-CPSI pain domain was -2.31 (95% CI -3.4, -1.21), urinary domain -1.12 (95% CI -1.62, -0.62), quality of life domain -1.67 (95% CI -2.38, -0.96); p<0.001 for all. Qmax mean change from baseline was 0.68 (95% CI -0.85, 2.22, p=0.38). Systematic review showed no significant changes in pain, measured by VAS or other scores, IPSS and PVR.
Conclusions: Placebo significantly affected the subjective parameters (NIH-CPSI) and had a minor effect on different measurements of pain, whereas there was no long-term effect on IPSS and the objective measurements (Qmax, PVR).
Source of Funding: None


.jpg)
.jpg)