Back
Poster, Podium & Video Sessions
Moderated Poster
MP39: Health Services Research: Value of Care: Cost and Outcomes Measures
MP39-13: Cost-effectiveness of Neoadjuvant Chemotherapy Regimens Prior to Radical Cystectomy for Muscle-Invasive Bladder Cancer
Sunday, May 15, 2022
8:45 AM – 10:00 AM
Location: Room 228
Daniel D. Joyce*, Rochester, MN, Kevin M. Wymer, Scottsdale, AZ, Vidit Sharma, James P. Moriarty, Bijan J. Borah, Rochester, MN, Daniel M. Geynisman, Elizabeth R. Plimack, Philadelphia, PA, Brian A. Costello, Lance C. Pagliaro, Stephen A. Boorjian, Rochester, MN

Daniel Joyce, MD
Mayo Clinic, Rochester, MN
Poster Presenter(s)
Introduction: A recent phase III randomized trial (VESPER) demonstrated superior pathologic endpoints of tumor downstaging as well as improved progression free (PFS) and overall survival (OS) with 6 cycles of neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (ddMVAC) versus 4 cycles of gemcitabine and cisplatin (GC) before radical cystectomy (RC) for muscle-invasive bladder cancer (MIBC) but at increased toxicity. Herein, we assess the cost-effectiveness of these regimens.
Methods: Cost-effectiveness analysis of each neoadjuvant chemotherapy was performed using a decision-analytic Markov model. Probabilities of toxicity, progression, and survival were derived from published or abstract-presented VESPER trial data. Utility values were obtained from the literature. Analyses were performed with a 3-month cycle length for 3-year, 10-year, and lifetime horizons using a 3% discount rate for cost and utilities. Primary outcomes were 2021 Medicare costs, effectiveness in terms of quality adjusted life years (QALY), and incremental cost-effectiveness ratio (ICER) with a willingness to pay threshold of $100,000 per QALY. One-way and probabilistic sensitivity analyses were performed to evaluate the robustness of the model.
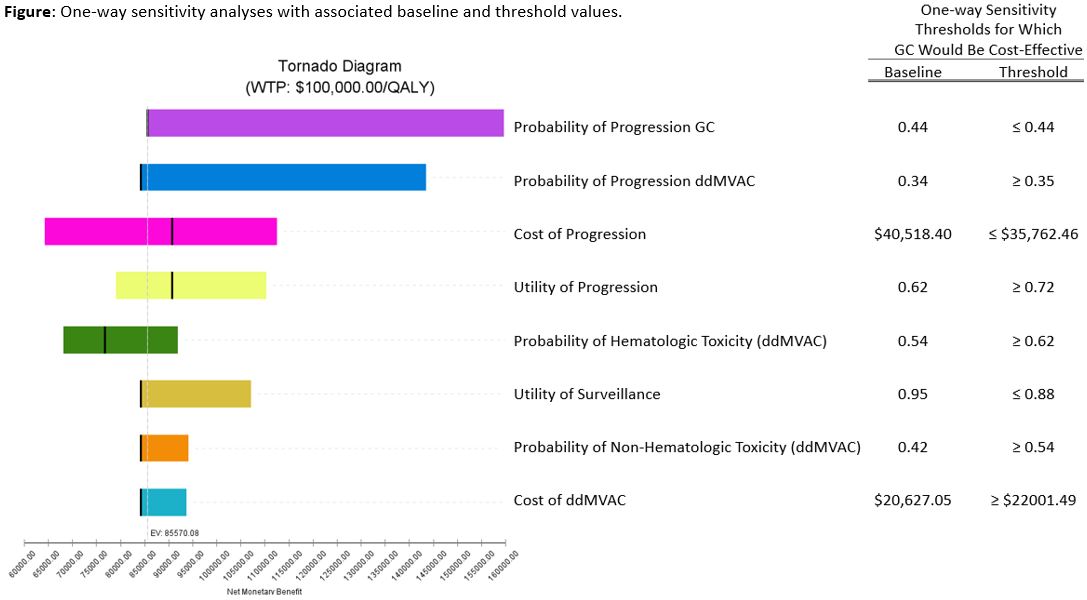
Results: At 3 years, mean costs and effectiveness per patient were $90,048.88 and 1.76 QALYs for ddMVAC vs. $80,166.57 and 1.64 QALYs for GC. Neoadjuvant ddMVAC was found to be more cost-effective than GC at 3 years (ICER $87,794/QALY), 10 years (ICER $16,919/QALY), and lifetime (ICER $17,882/QALY) horizons. One-way sensitivity analysis identified ddMVAC cost-effectiveness thresholds for hematologic and non-hematologic toxicity at >62% and >54% respectively (Figure). Notably, a disease progression advantage of 9.4% at 3 years was identified as necessary for ddMVAC to remain cost-effective. Probabilistic sensitivity analysis favored ddMVAC in 54% of 100,000 microsimulations.
Conclusions: While both regimens comprise of generic treatments, ddMVAC was more cost-effective than GC in the neoadjuvant setting for MIBC at 3-year, 10-year, and lifetime horizons. These data, together with the improved PFS and OS, support use of this regimen prior to RC.
Source of Funding: None
Methods: Cost-effectiveness analysis of each neoadjuvant chemotherapy was performed using a decision-analytic Markov model. Probabilities of toxicity, progression, and survival were derived from published or abstract-presented VESPER trial data. Utility values were obtained from the literature. Analyses were performed with a 3-month cycle length for 3-year, 10-year, and lifetime horizons using a 3% discount rate for cost and utilities. Primary outcomes were 2021 Medicare costs, effectiveness in terms of quality adjusted life years (QALY), and incremental cost-effectiveness ratio (ICER) with a willingness to pay threshold of $100,000 per QALY. One-way and probabilistic sensitivity analyses were performed to evaluate the robustness of the model.
Results: At 3 years, mean costs and effectiveness per patient were $90,048.88 and 1.76 QALYs for ddMVAC vs. $80,166.57 and 1.64 QALYs for GC. Neoadjuvant ddMVAC was found to be more cost-effective than GC at 3 years (ICER $87,794/QALY), 10 years (ICER $16,919/QALY), and lifetime (ICER $17,882/QALY) horizons. One-way sensitivity analysis identified ddMVAC cost-effectiveness thresholds for hematologic and non-hematologic toxicity at >62% and >54% respectively (Figure). Notably, a disease progression advantage of 9.4% at 3 years was identified as necessary for ddMVAC to remain cost-effective. Probabilistic sensitivity analysis favored ddMVAC in 54% of 100,000 microsimulations.
Conclusions: While both regimens comprise of generic treatments, ddMVAC was more cost-effective than GC in the neoadjuvant setting for MIBC at 3-year, 10-year, and lifetime horizons. These data, together with the improved PFS and OS, support use of this regimen prior to RC.
Source of Funding: None

.jpg)
.jpg)