Back
Poster, Podium & Video Sessions
Moderated Poster
MP43: Prostate Cancer: Localized: Active Surveillance
MP43-13: Interlaboratory Gleason Grading Variation Affects Treatment: a Dutch Historic Cohort Study in 30,509 Prostate Cancer Patients
Sunday, May 15, 2022
10:30 AM – 11:45 AM
Location: Room 222
Rachel Flach*, Carmen van Dooijeweert, Katja Aben, Britt Suelmann, Peter-Paul Willemse, Paul van Diest, Richard Meijer, Utrecht, Netherlands
Poster Presenter(s)
Introduction: Substantial variation in Gleason grading (GG) of prostate cancer (PCa) exists between Dutch pathology laboratories. This study investigates its impact on treatment strategies.
Methods: Pathology reports of prostate needle biopsies and clinical data of PCa patients diagnosed between 2017-2019 were retrieved from the Dutch nationwide network and registry of histo- and cytopathology (PALGA) and The Netherlands Cancer Registry. The impact of grading variation between pathology laboratories on treatment strategy was investigated for patients for whom GG was decisive in treatment choice, based on the D’Amico risk stratification. First, we evaluated the effect of grading practice on active treatment (AT) versus active surveillance (AS) in patients with PSA <10 ng/mL and cT1c/cT2a disease. Second, we assessed the association of grading practice with performance of pelvic lymph node dissection (PLND) in patients with PSA 10-20 ng/mL or cT2b disease. We investigated the relation between laboratories’ grading practices (low-, average-, or high-grading) and AT or PLND using multivariable logistic regression.
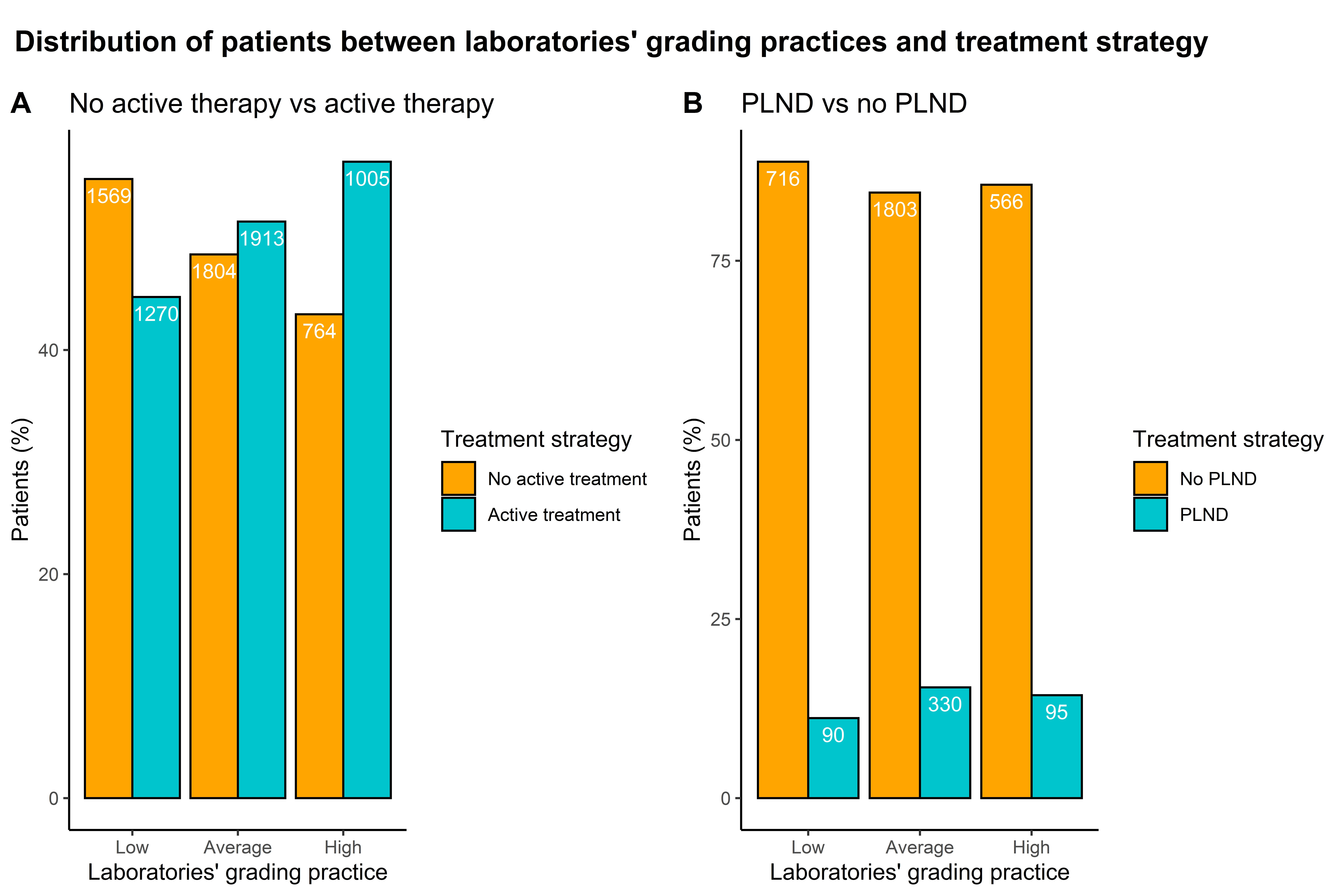
Results: We included 30,503 patients. We identified 11,925 patients (39%) for whom GG was decisive in treatment strategy. AT was performed significantly less often in patients diagnosed by laboratories that graded consistently lower than average (OR=0.79, CI95=0.71-0.87). Conversely, patients diagnosed in laboratories that graded higher than average received AT significantly more often (OR=1.16, CI95=1.03-1.30). PLND was only performed significantly less often in patients diagnosed by low- versus average-grading laboratories (OR=0.63, CI95=0.48-0.81).
Conclusions: Our study shows the importance of consistency in GG for clinical decision-making in a Dutch population-based cohort. The chance that a patient undergoes AT or PLND, depends on laboratory grading practice in a substantial number of patients. As this likely influences patient prognosis and outcome, standardisation of GG is necessary to prevent sub-optimal patient outcome.
Source of Funding: This research was funded by Quality Foundation of the Dutch Association of Medical Specialists (SKMS), Astellas Pharma BV and Pfizer BV”
Methods: Pathology reports of prostate needle biopsies and clinical data of PCa patients diagnosed between 2017-2019 were retrieved from the Dutch nationwide network and registry of histo- and cytopathology (PALGA) and The Netherlands Cancer Registry. The impact of grading variation between pathology laboratories on treatment strategy was investigated for patients for whom GG was decisive in treatment choice, based on the D’Amico risk stratification. First, we evaluated the effect of grading practice on active treatment (AT) versus active surveillance (AS) in patients with PSA <10 ng/mL and cT1c/cT2a disease. Second, we assessed the association of grading practice with performance of pelvic lymph node dissection (PLND) in patients with PSA 10-20 ng/mL or cT2b disease. We investigated the relation between laboratories’ grading practices (low-, average-, or high-grading) and AT or PLND using multivariable logistic regression.
Results: We included 30,503 patients. We identified 11,925 patients (39%) for whom GG was decisive in treatment strategy. AT was performed significantly less often in patients diagnosed by laboratories that graded consistently lower than average (OR=0.79, CI95=0.71-0.87). Conversely, patients diagnosed in laboratories that graded higher than average received AT significantly more often (OR=1.16, CI95=1.03-1.30). PLND was only performed significantly less often in patients diagnosed by low- versus average-grading laboratories (OR=0.63, CI95=0.48-0.81).
Conclusions: Our study shows the importance of consistency in GG for clinical decision-making in a Dutch population-based cohort. The chance that a patient undergoes AT or PLND, depends on laboratory grading practice in a substantial number of patients. As this likely influences patient prognosis and outcome, standardisation of GG is necessary to prevent sub-optimal patient outcome.
Source of Funding: This research was funded by Quality Foundation of the Dutch Association of Medical Specialists (SKMS), Astellas Pharma BV and Pfizer BV”


.jpg)
.jpg)
