Back
Poster, Podium & Video Sessions
Moderated Poster
MP46: Penile & Testicular Cancer I
MP46-10: Elucidating therapeutic options for treatment-naïve and cisplatin-resistant testicular cancer
Sunday, May 15, 2022
1:00 PM – 2:15 PM
Location: Room 222
Julie-Ann Cavallo, Daniel Ranti*, Elliot Merritt, Anna Tocheva, John P. Sfakianos, Benjamin Hopkins, New York, NY

Daniel Ranti, BS
Icahn School of Medicine
Poster Presenter(s)
Introduction: Platinum-based chemotherapy is the standard of care (SOC) for patients with advanced testicular cancer (TC). However, outcomes for platinum-refractory tumors remain poor. This project seeks to use functional diagnostic approach to both predict therapeutic response for patients with testicular cancer and evaluate the utility of alternate therapeutic strategies that may improve responses for patients with platinum-refractory disease.
Methods: Using an IRB-approved protocol, we obtained tissue from platinum-sensitive and -resistant TC patients. Tissue specimens were obtained from 13 tumors: 10 primary, 2 post-treatment, and 1 lymph node. To functionally model therapeutic responses for patients with TC, we used our RAPiDS (Rapid Assessment of Patient Drug Sensitivity) screens on primary patient-derived organoids (PDOs) to stratify patient response to SOC therapies and evaluate the relative sensitivity of the tumors to alternative therapeutic regimens. For these studies, we conducted screens with nine therapeutics in a clinically relevant range of concentrations to extract correlative drug response data within two weeks from dissociation.
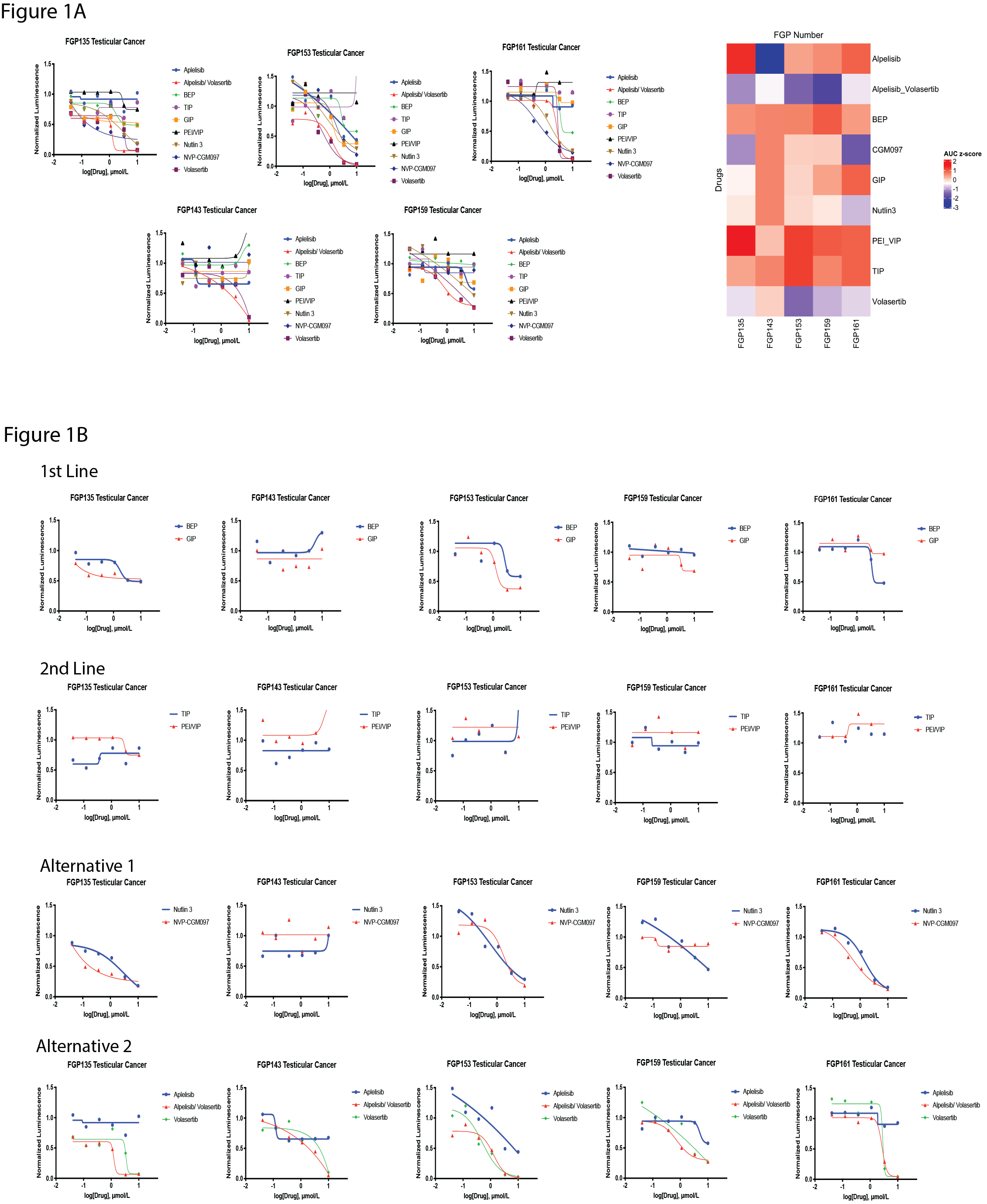
Results: Using the RAPiDS protocol, we developed and evaluated drug sensitivities in five PDOs (Figure 1A). Normalized luminescence from Cell-TiterGlo cell viability assays were used to determine Area Under the Curve (AUC) as a function of drug concentration (Figure 1A and B). Heatmaps show the median centered z-score for these AUC values across the therapeutics screened in each patient to gauge relative sensitivity (Figure 1A). All of our screened TC cases showed sensitivity to one or more alternative therapies tested, regardless of PDO response to first- or second-line SOC (Figure 1B).
Conclusions: This data suggests that RAPiDS could be used on TC PDOs to help guide the selection of treatment options within clinically relevant timelines, which has the potential to be useful, especially in metastatic and rare cancer settings where therapeutic options are limited. Additionally, we are currently investigating the utility of RAPiDS in refractory and metastatic TC.
Source of Funding: Mount Sinai School of Medicine seed funds.
Methods: Using an IRB-approved protocol, we obtained tissue from platinum-sensitive and -resistant TC patients. Tissue specimens were obtained from 13 tumors: 10 primary, 2 post-treatment, and 1 lymph node. To functionally model therapeutic responses for patients with TC, we used our RAPiDS (Rapid Assessment of Patient Drug Sensitivity) screens on primary patient-derived organoids (PDOs) to stratify patient response to SOC therapies and evaluate the relative sensitivity of the tumors to alternative therapeutic regimens. For these studies, we conducted screens with nine therapeutics in a clinically relevant range of concentrations to extract correlative drug response data within two weeks from dissociation.
Results: Using the RAPiDS protocol, we developed and evaluated drug sensitivities in five PDOs (Figure 1A). Normalized luminescence from Cell-TiterGlo cell viability assays were used to determine Area Under the Curve (AUC) as a function of drug concentration (Figure 1A and B). Heatmaps show the median centered z-score for these AUC values across the therapeutics screened in each patient to gauge relative sensitivity (Figure 1A). All of our screened TC cases showed sensitivity to one or more alternative therapies tested, regardless of PDO response to first- or second-line SOC (Figure 1B).
Conclusions: This data suggests that RAPiDS could be used on TC PDOs to help guide the selection of treatment options within clinically relevant timelines, which has the potential to be useful, especially in metastatic and rare cancer settings where therapeutic options are limited. Additionally, we are currently investigating the utility of RAPiDS in refractory and metastatic TC.
Source of Funding: Mount Sinai School of Medicine seed funds.


.jpg)
.jpg)