Back
Poster, Podium & Video Sessions
Moderated Poster
MP47: Kidney Cancer: Epidemiology & Evaluation/Staging/Surveillance III
MP47-20: Universal Germline Genetic Testing of Patients with Newly Diagnosed Upper Tract Urothelial Carcinoma: An Interim Analysis
Sunday, May 15, 2022
2:45 PM – 4:00 PM
Location: Room 228
Craig Labbate*, Donika Saporito, Maureen Mork, Eduardo Vilar Sanchez, Eric Jonasch, Mehrad Adibi, Surena Matin, Houston, TX

Craig Labbate, MD
Urologic Oncology Fellow
UT MD Anderson Cancer Center
Poster Presenter(s)
Introduction: Upper tract urothelial carcinoma (UTUC) is a known Lynch Syndrome associated malignancy. Screening for LS after diagnosis by immunohistochemistry can be limited by low volume of biopsied tissue. At one tertiary cancer center, we have referred all new diagnoses of UTUC for germline genetic testing.
Methods: All new patients with a diagnosis of upper tract urothelial carcinoma seen between 9/2020 to 10/2021 were referred for genetic counseling and detailed family history assessment. If willing, patients underwent germline genetic testing of MLH1, MSH2, MHS6, PMS2, EPCAM, BRCA1 and BRCA2 by a CLIA certified lab (Invitae, San Francisco, CA). Both direct sequencing and copy number variations were assessed.
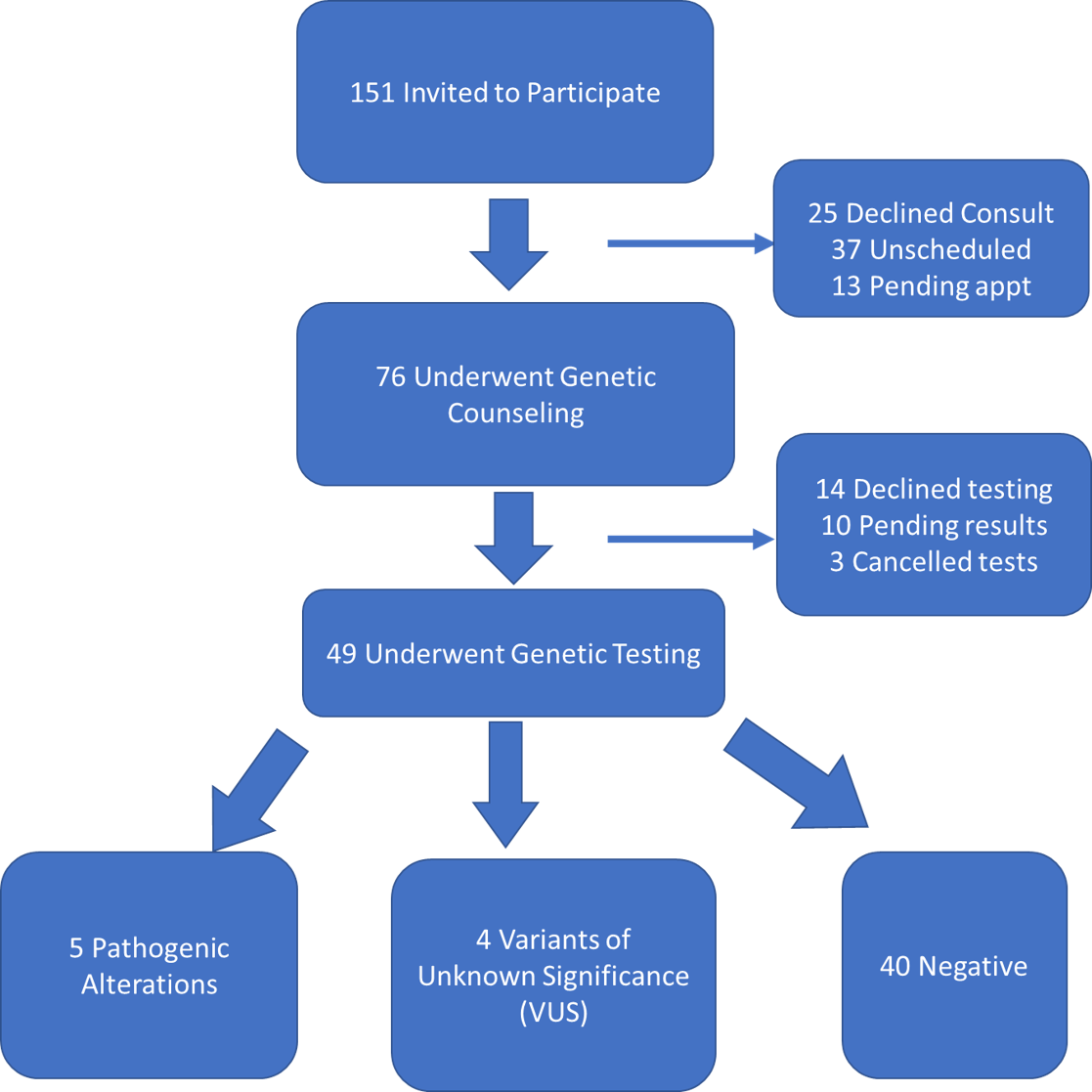
Results: One hundred fifty-one patients were offered genetic testing of which 76 underwent genetic counseling and 49 proceeded to genetic testing. Of those who underwent testing, there were 5 (10%) detected pathogenic variants (4 in MMR genes [3 MSH6, 1 MSH2], 1 BRCA1). Additionally, 4 (8%) patients had variants of unknown significance (2 MSH2, 1 MSH6, 1 BRCA2). Forty patients had negative genetic testing. No patient, including all 7 with MMR alterations, met strict Amsterdam II criteria for Lynch Syndrome genetic testing by family history.
Conclusions: Universal germline genetic testing of patients with newly diagnosed upper tract urothelial carcinoma revealed that 14% of patients harbored pathogenic or VUS alterations in MMR and 4% within BRCA genes. Universal germline testing may identify a higher incidence of Lynch Syndrome than previously reported in prescreened cohorts.
Source of Funding: Pam and Jim Harris UTUC Lynch Syndrome Screening Fund
Methods: All new patients with a diagnosis of upper tract urothelial carcinoma seen between 9/2020 to 10/2021 were referred for genetic counseling and detailed family history assessment. If willing, patients underwent germline genetic testing of MLH1, MSH2, MHS6, PMS2, EPCAM, BRCA1 and BRCA2 by a CLIA certified lab (Invitae, San Francisco, CA). Both direct sequencing and copy number variations were assessed.
Results: One hundred fifty-one patients were offered genetic testing of which 76 underwent genetic counseling and 49 proceeded to genetic testing. Of those who underwent testing, there were 5 (10%) detected pathogenic variants (4 in MMR genes [3 MSH6, 1 MSH2], 1 BRCA1). Additionally, 4 (8%) patients had variants of unknown significance (2 MSH2, 1 MSH6, 1 BRCA2). Forty patients had negative genetic testing. No patient, including all 7 with MMR alterations, met strict Amsterdam II criteria for Lynch Syndrome genetic testing by family history.
Conclusions: Universal germline genetic testing of patients with newly diagnosed upper tract urothelial carcinoma revealed that 14% of patients harbored pathogenic or VUS alterations in MMR and 4% within BRCA genes. Universal germline testing may identify a higher incidence of Lynch Syndrome than previously reported in prescreened cohorts.
Source of Funding: Pam and Jim Harris UTUC Lynch Syndrome Screening Fund


.jpg)
.jpg)