Back
Poster, Podium & Video Sessions
Moderated Poster
MP48: Prostate Cancer: Advanced (including Drug Therapy) III
MP48-02: Persistent Testosterone Suppression After Cessation of Androgen Deprivation Therapy for Localized Prostate Cancer
Sunday, May 15, 2022
2:45 PM – 4:00 PM
Location: Room 225
Jessica Delgado*, Miami, FL, Jesse Ory, Nova Scotia , Canada, Joshua Bitran, Ruben Blachman Braun, Sirpi Nackeeran, Ranjith Ramasamy, Miami, FL

Jessica Delgado, MD,MS
University of Miami/ Jackson Health System
Poster Presenter(s)
Introduction: Introduction
ADT plays a fundamental role in the treatment of localized prostate cancer. However, there is limited data regarding testosterone recovery in men who have received ADT for localized prostate cancer. Identification of T recovery profiles associated with ADT will facilitate personalization of ADT regimens and guide future treatment strategies to minimize the risk of T deficiency in patients following treatment for prostate cancer.
Objective
Temporary use of Androgen Deprivation Therapy (ADT) is a cornerstone in the treatment of localized prostate cancer. However, the ability for testosterone to recover after ADT is not well understood. The aim of this study was to investigate testosterone recovery in men with localized prostate cancer following ADT.
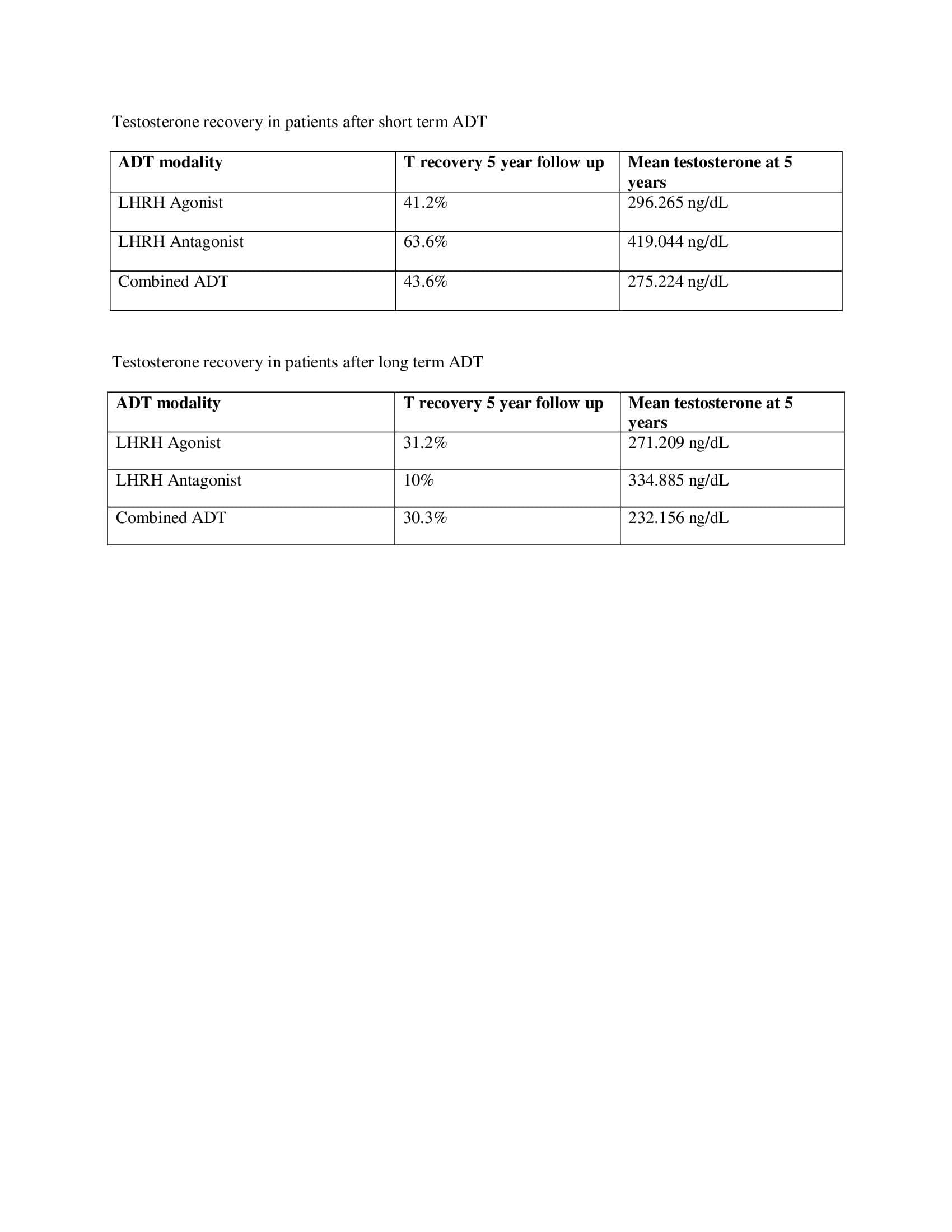
Methods: A global federated health research network (TriNetX) was used to identify men with a diagnosis of prostate cancer who underwent temporary use of ADT. Three cohorts were identified: Men who received LHRH antagonists, LHRH agonists, and men who received combined ADT (LHRH agonist and antiandrogens). Further stratification was based on treatment duration of 6 or 18 months to compare T recovery profiles 5 years after ADT cessation.
Results: A total of 17,884 men received LHRH agonists alone, 12,767 men received combined ADT, and 628 men received LHRH antagonist therapy alone. Eugondal mean baseline T level (>300 ng/dL) prior to starting ADT was an inclusion criterion for all men. Five years after ADT cessation, 36% of patients who received LHRH agonists recovered eugondal T levels, 26% recovered after LHRH antagonist therapy, and 36.8% recovered after combined ADT.
Overall, more than half of men who received ADT failed to recover eugondal T levels even 5 years after treatment cessation.
Conclusions: Five years after ADT cessation for localized prostate cancer, incomplete testosterone recovery persists in >50% of the men. Since, testosterone deficiency will lead to metabolically adverse changes in body composition, increased insulin resistance, impaired bone health, and poor quality of life, serum T levels need to be closely followed in men receiving ADT.
Source of Funding: Funding to access the TriNetX database was provided in part by an educational grant from Acerus Pharmaceuticals. Acerus Pharmaceuticals was not involved in the planning, design, writing or any other aspect of this project.
ADT plays a fundamental role in the treatment of localized prostate cancer. However, there is limited data regarding testosterone recovery in men who have received ADT for localized prostate cancer. Identification of T recovery profiles associated with ADT will facilitate personalization of ADT regimens and guide future treatment strategies to minimize the risk of T deficiency in patients following treatment for prostate cancer.
Objective
Temporary use of Androgen Deprivation Therapy (ADT) is a cornerstone in the treatment of localized prostate cancer. However, the ability for testosterone to recover after ADT is not well understood. The aim of this study was to investigate testosterone recovery in men with localized prostate cancer following ADT.
Methods: A global federated health research network (TriNetX) was used to identify men with a diagnosis of prostate cancer who underwent temporary use of ADT. Three cohorts were identified: Men who received LHRH antagonists, LHRH agonists, and men who received combined ADT (LHRH agonist and antiandrogens). Further stratification was based on treatment duration of 6 or 18 months to compare T recovery profiles 5 years after ADT cessation.
Results: A total of 17,884 men received LHRH agonists alone, 12,767 men received combined ADT, and 628 men received LHRH antagonist therapy alone. Eugondal mean baseline T level (>300 ng/dL) prior to starting ADT was an inclusion criterion for all men. Five years after ADT cessation, 36% of patients who received LHRH agonists recovered eugondal T levels, 26% recovered after LHRH antagonist therapy, and 36.8% recovered after combined ADT.
Overall, more than half of men who received ADT failed to recover eugondal T levels even 5 years after treatment cessation.
Conclusions: Five years after ADT cessation for localized prostate cancer, incomplete testosterone recovery persists in >50% of the men. Since, testosterone deficiency will lead to metabolically adverse changes in body composition, increased insulin resistance, impaired bone health, and poor quality of life, serum T levels need to be closely followed in men receiving ADT.
Source of Funding: Funding to access the TriNetX database was provided in part by an educational grant from Acerus Pharmaceuticals. Acerus Pharmaceuticals was not involved in the planning, design, writing or any other aspect of this project.


.jpg)
.jpg)