Back
Poster, Podium & Video Sessions
Moderated Poster
MP48: Prostate Cancer: Advanced (including Drug Therapy) III
MP48-06: Adverse events related to Darolutamide treatment: analysis of “real life” data from EudraVigilance (EV) and the Food and Drug Administration (FDA) database entries
Sunday, May 15, 2022
2:45 PM – 4:00 PM
Location: Room 225
Antonio Cicione*, Rome, Italy, Antonio Nacchia, Rionero in Vulture, Italy, Beatrice Turchi, Carmen Gravina, Giacomo Gallo, Alessandro Guercio, Lorenzo Maria Rovesti, Giorgio Guarnotta, Elisa Mancini, Jordi Stira, Sara Riolo, Antonio Franco, Olivia Alessandra Voglino, Valeria Baldassarri, Simone D'Annunzio, Rome, Italy, Ferdinando Di Giacomo, Giuseppe Disabato, Alfredo Tartarone, Rionero in Vulture, Italy, Cosimo De Nunzio, Andrea Tubaro, Rome, Italy
- AC
Poster Presenter(s)
Introduction: Darolutamide, an oral androgen receptor inhibitor, has been approved for treating nonmetastatic castration-resistant prostate cancer (nmCRPC), based on significant improvements in metastasis-free survival (MFS) in the ARAMIS clinical trial. Aim of our study was to analyse adverse events (AEs) associated with darolutamide using real life data from EV and the FAERS databases.
Methods: EV database in European Economic Area and the FAERS database were queried to identify darolutamide AEs occurred from 30th July 2019 to 1st October 2021. AEs were recorded in according to category and severity. Real-life data was compared to Aramis registry study. Furthermore, AEs reported in EV database were categorized by patient’s age into more or less than 85 years. Pooled relative risk (PRR) was used to compare groups.
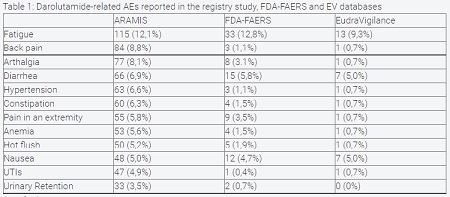
Results: On EV database 139 AEs related to Darolutamide were identified, of which 54 (39%) were serious and 2 (0,01%) were deaths. On FDA FAERS database 257 adverse events related to Darolutamide were reported with 137 (53%) serious events and 8 (3%) death cases. On registry study, 794 AEs were reported, with serious AEs occurring in 24.8% of patients in the Darolutamide group and with 1 death related to trial regimen. Fatigue was the most common AE reported in all three datasets, respectively 115 events (12,1%) in the registry study, 33 events (12,8%) in FDA-FAERS and 13 events (9,3%) in EV. However, no significative differences were found in all three datasets for fatigue, diarrhea and nausea (PRR 0,80 – 1,50), p>0,05. Higher rates of back pain, arthalgia, hypertension, constipation, pain in an extremity, anemia, hot flush, UTIs and urinary retention were found in the registry study when compared to EV and FDA-FAERS databases (PRR 1,50-7,00, p<0,05). Unfortunately, no information on the number of patients under treatment was reported in the EV database.
Conclusions: According to our results Darolutamide is safe in a real-life scenario and the most frequent side effect is fatigue. Although up to now there are few reports in both real-life databases, these data are encouraging for clinicians using darolutamide in every day clinical practice.
Source of Funding: None
Methods: EV database in European Economic Area and the FAERS database were queried to identify darolutamide AEs occurred from 30th July 2019 to 1st October 2021. AEs were recorded in according to category and severity. Real-life data was compared to Aramis registry study. Furthermore, AEs reported in EV database were categorized by patient’s age into more or less than 85 years. Pooled relative risk (PRR) was used to compare groups.
Results: On EV database 139 AEs related to Darolutamide were identified, of which 54 (39%) were serious and 2 (0,01%) were deaths. On FDA FAERS database 257 adverse events related to Darolutamide were reported with 137 (53%) serious events and 8 (3%) death cases. On registry study, 794 AEs were reported, with serious AEs occurring in 24.8% of patients in the Darolutamide group and with 1 death related to trial regimen. Fatigue was the most common AE reported in all three datasets, respectively 115 events (12,1%) in the registry study, 33 events (12,8%) in FDA-FAERS and 13 events (9,3%) in EV. However, no significative differences were found in all three datasets for fatigue, diarrhea and nausea (PRR 0,80 – 1,50), p>0,05. Higher rates of back pain, arthalgia, hypertension, constipation, pain in an extremity, anemia, hot flush, UTIs and urinary retention were found in the registry study when compared to EV and FDA-FAERS databases (PRR 1,50-7,00, p<0,05). Unfortunately, no information on the number of patients under treatment was reported in the EV database.
Conclusions: According to our results Darolutamide is safe in a real-life scenario and the most frequent side effect is fatigue. Although up to now there are few reports in both real-life databases, these data are encouraging for clinicians using darolutamide in every day clinical practice.
Source of Funding: None


.jpg)
.jpg)