Back
Poster, Podium & Video Sessions
Moderated Poster
MP48: Prostate Cancer: Advanced (including Drug Therapy) III
MP48-19: Risk of metabolic and cardiovascular adverse events with abiraterone or enzalutamide among men with advanced prostate cancer
Sunday, May 15, 2022
2:45 PM – 4:00 PM
Location: Room 225
Lillian Lai*, Mary Oerline, Megan Caram, Phoebe Tsao, Samuel Kaufman, Brent Hollenbeck, Vahakn Shahinian, Ann Arbor, MI

Lillian Y. Lai, MD,MS
University of Michigan
Poster Presenter(s)
Introduction: Abiraterone and enzalutamide, used with androgen deprivation, are the most common treatments for men with advanced prostate cancer. Both have anti-androgenic properties and abiraterone is prescribed with prednisone, which may contribute to metabolic or cardiovascular adverse events. To understand the safety of abiraterone and enzalutamide use in real-world settings, we examined the association between the use of these agents and the risk of metabolic or cardiovascular events while on treatment.
Methods: 56,230 men with advanced prostate cancer were identified in a 20% sample of the 2011-2017 national Medicare claims. Abiraterone or enzalutamide use was analyzed as a time-dependent covariate. The primary outcome was the occurrence of a major metabolic or cardiovascular event, defined as an emergency room visit or hospitalization associated with a primary diagnosis of diabetes, hypertension, or cardiovascular disease. The secondary outcome was the occurrence of a minor metabolic or cardiovascular event, defined as an outpatient visit associated with a primary diagnosis of the aforementioned conditions. Men who experienced an event of interest in the 12 months prior to the study start date were excluded. The risks of primary and secondary composite outcomes were assessed using Cox regression. The occurrence of adverse events by specific diagnoses was also examined individually.
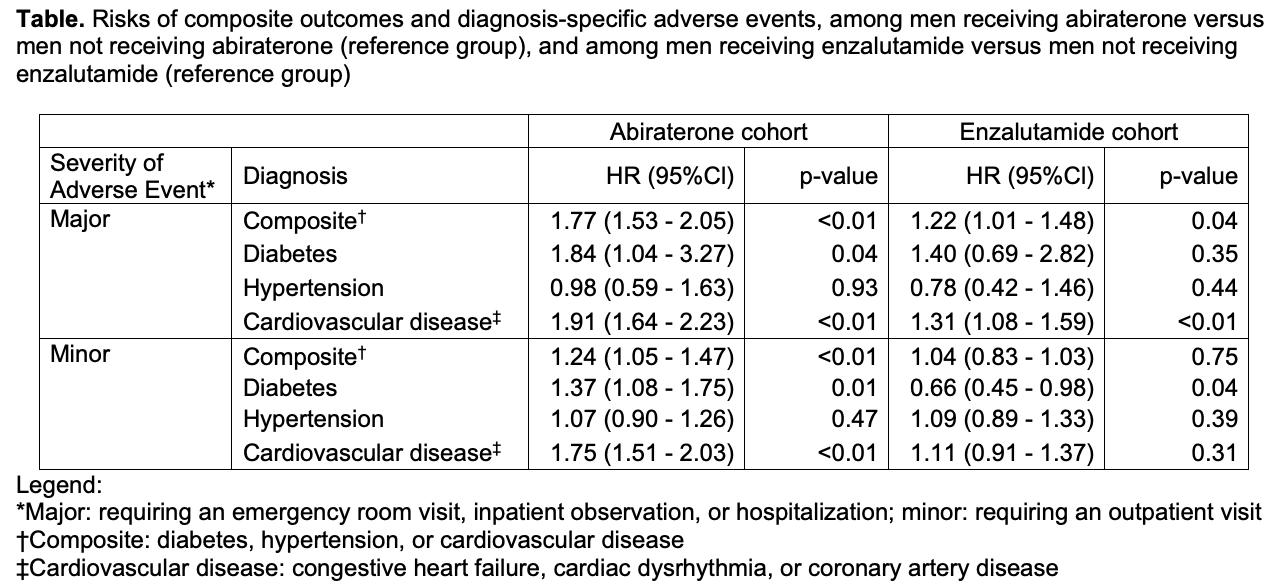
Results: Per unadjusted incidence rates, men receiving abiraterone had 1 additional emergency room visit/hospitalization due to a cardiovascular event for every 29 men treated (median time on drug: 4.7 months). Compared to men not receiving abiraterone, men receiving the drug were at increased risk of both a major composite adverse event (HR 1.77 95% CI 1.53-2.05 p<0.01) and a minor composite adverse event (HR 1.24 95% CI 1.05-1.47 p=0.01) (Table). Compared to men not receiving enzalutamide, men receiving enzalutamide were at an increased risk of a major composite adverse event (HR 1.22 95% CI 1.01-1.48 p=0.04) but not a minor composite adverse event (HR 1.04 95% CI 0.83-1.30 p=0.75).
Conclusions: Treatment with abiraterone or enzalutamide is associated with increased risk of major metabolic or cardiovascular events in real-world settings.
Source of Funding: AHRQ R01HS027507
NCI T32 CA180984
Prostate Cancer Foundation
Methods: 56,230 men with advanced prostate cancer were identified in a 20% sample of the 2011-2017 national Medicare claims. Abiraterone or enzalutamide use was analyzed as a time-dependent covariate. The primary outcome was the occurrence of a major metabolic or cardiovascular event, defined as an emergency room visit or hospitalization associated with a primary diagnosis of diabetes, hypertension, or cardiovascular disease. The secondary outcome was the occurrence of a minor metabolic or cardiovascular event, defined as an outpatient visit associated with a primary diagnosis of the aforementioned conditions. Men who experienced an event of interest in the 12 months prior to the study start date were excluded. The risks of primary and secondary composite outcomes were assessed using Cox regression. The occurrence of adverse events by specific diagnoses was also examined individually.
Results: Per unadjusted incidence rates, men receiving abiraterone had 1 additional emergency room visit/hospitalization due to a cardiovascular event for every 29 men treated (median time on drug: 4.7 months). Compared to men not receiving abiraterone, men receiving the drug were at increased risk of both a major composite adverse event (HR 1.77 95% CI 1.53-2.05 p<0.01) and a minor composite adverse event (HR 1.24 95% CI 1.05-1.47 p=0.01) (Table). Compared to men not receiving enzalutamide, men receiving enzalutamide were at an increased risk of a major composite adverse event (HR 1.22 95% CI 1.01-1.48 p=0.04) but not a minor composite adverse event (HR 1.04 95% CI 0.83-1.30 p=0.75).
Conclusions: Treatment with abiraterone or enzalutamide is associated with increased risk of major metabolic or cardiovascular events in real-world settings.
Source of Funding: AHRQ R01HS027507
NCI T32 CA180984
Prostate Cancer Foundation


.jpg)
.jpg)