Back
Poster, Podium & Video Sessions
Moderated Poster
MP49: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Basic Research & Pathophysiology
MP49-17: Pudendal Nerve Mapping And The Use Of Urodynamics To Examine The Effects Of Pudendal Neuromodulation
Sunday, May 15, 2022
4:30 PM – 5:45 PM
Location: Room 228
Priyanka Gupta*, Po-Ju Chen, Amador Lagunas, Luis C. Ruiz, Anagha Kotkar, Gaurang Shah, Scott F. Lempka, Tim M. Bruns, Ann Arbor, MI
- PG
Priyanka Gupta, MD (she/her/hers)
Associate Professor of Urology
University of Michigan
Poster Presenter(s)
Introduction: Pudendal neuromodulation (PNM) is effective for refractory voiding dysfunction and pelvic pain. The trajectory of the pudendal nerve (PN) branches and the effects of PNM on voiding are incompletely understood. The objective of this study is to use magnetic resonance (MR) nerve imaging to understand the anatomical pathway of the PN, perform patient specific computational modeling of neuromodulation, and examine the effects of PNM on voiding through urodynamics.
Methods: A PNM patient underwent MR nerve imaging prior to implant to delineate PN anatomy and generate a patient-specific model to characterize the relative recruitment order of the genital, perineal, and rectal fascicles within the PN. Stimulation selectivity was calculated from OR testing and modeling. A Manoscan catheter, which contains 7-mm pressure sensor electrodes along the functional length of the catheter, was used to measure pressures along the bladder, bladder neck, and proximal and distal urethra. Post-operative cystometrogram (CMG) was performed to evaluate electrode stimulation and effects stimulation parameters on end-organ recruitment. Pre and post-operative clinical outcomes were collected.
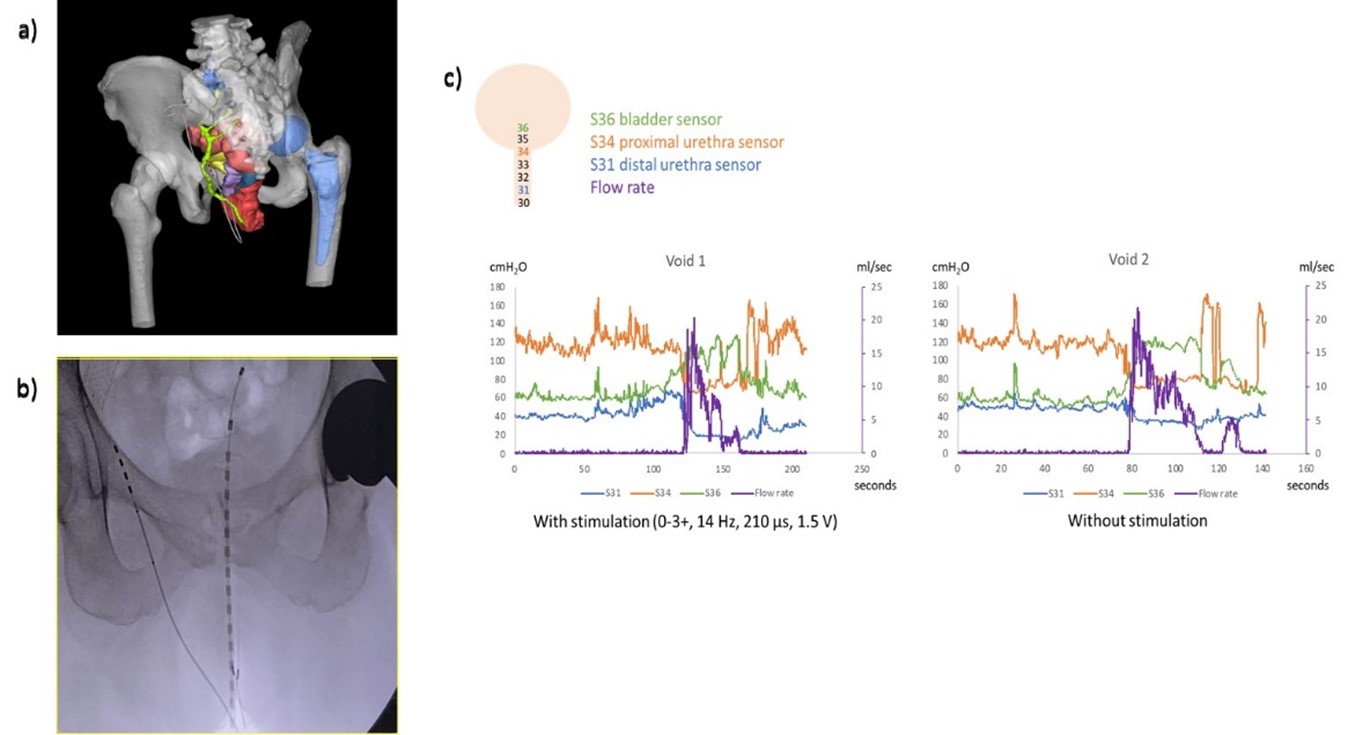
Results: MR nerve imaging generated a patient-specific model (Figure 1a). OR testing was performed using the Manoscan catheter per urethra. (Figure 1b) Some electrode combinations recruited the urethra before the external anal sphincter. The patient noted significant improvement in her urinary and pain symptoms. On standardized questionnaires: AUA Symptom Score improved from 13 to 6, QOL improved from unhappy to pleased, Female Genitourinary Pain Index improved from 21 to 7. During follow-up CMG, subjective urgency symptoms subsided each time the PNM device was turned on during filling. No changes were noted in detrusor pressures. During voiding the Manoscan catheter detected distinct pressure changes in the detrusor followed by the proximal urethra and distal urethra. (Figure 1c)
Conclusions: Initial data demonstrates the ability to generate a patient specific map of the PN and the effects of stimulation on voiding both in the OR and during CMG testing. This methodology will further our understanding of the PN anatomy and the effects of PNM on voiding dynamics.
Source of Funding: NIH SPARC OT2OD028191
Methods: A PNM patient underwent MR nerve imaging prior to implant to delineate PN anatomy and generate a patient-specific model to characterize the relative recruitment order of the genital, perineal, and rectal fascicles within the PN. Stimulation selectivity was calculated from OR testing and modeling. A Manoscan catheter, which contains 7-mm pressure sensor electrodes along the functional length of the catheter, was used to measure pressures along the bladder, bladder neck, and proximal and distal urethra. Post-operative cystometrogram (CMG) was performed to evaluate electrode stimulation and effects stimulation parameters on end-organ recruitment. Pre and post-operative clinical outcomes were collected.
Results: MR nerve imaging generated a patient-specific model (Figure 1a). OR testing was performed using the Manoscan catheter per urethra. (Figure 1b) Some electrode combinations recruited the urethra before the external anal sphincter. The patient noted significant improvement in her urinary and pain symptoms. On standardized questionnaires: AUA Symptom Score improved from 13 to 6, QOL improved from unhappy to pleased, Female Genitourinary Pain Index improved from 21 to 7. During follow-up CMG, subjective urgency symptoms subsided each time the PNM device was turned on during filling. No changes were noted in detrusor pressures. During voiding the Manoscan catheter detected distinct pressure changes in the detrusor followed by the proximal urethra and distal urethra. (Figure 1c)
Conclusions: Initial data demonstrates the ability to generate a patient specific map of the PN and the effects of stimulation on voiding both in the OR and during CMG testing. This methodology will further our understanding of the PN anatomy and the effects of PNM on voiding dynamics.
Source of Funding: NIH SPARC OT2OD028191


.jpg)
.jpg)