Back
Poster, Podium & Video Sessions
Moderated Poster
MP53: Prostate Cancer: Detection & Screening V
MP53-16: The trends of complication rates and the role of aztreonam as a prophylactic antibiotic after prostate biopsy
Monday, May 16, 2022
7:00 AM – 8:15 AM
Location: Room 225
Sung Jin Kim*, Han Kyu Chae, Wook Nam, Kim Han Gwun, Park Jong Yeon, Gangneung-si, Korea, Republic of
- SK
Poster Presenter(s)
Introduction: An increase in the rate of complications after prostate biopsy due to an increase in antibiotic-resistant bacteria is a global problem. We report the safety of aztreonam as a prophylactic antibiotic in patients undergoing prostate biopsies.
Methods: Clinical data were obtained from 3,638 patients who underwent PB at our hospital from 1997 to 2019. We investigated the complication rates according to group of antibiotic regimens (Figure1). To analyse the incidence of complications according to the type of antibiotic prescribed, patients who experienced procedure-related complications within 2 weeks of the PB and were treated in the ER or in an outpatient setting were categorised as Cx1(complication1). Other definition of complication outcomes are described in Figure2.
Results: The clinical cohort in this study included 2,638 patients; 1,457 of these were administered oral quinolone and IV aztreonam. The rate of complications was significantly lower in patients who received oral quinolone and IV aztreonam than in those who received oral quinolone only (p=0.004). Two (0.1%) patients experienced febrile complications. The rate of Cx4 was significantly lower in the clinical cohort in 2018 and 2019 than in 2007, 2010, and 2011 (p < 0.01). The rate of Cx5 did not differ by year in the clinical cohort. The rate of Cx4 was significantly lower in the clinical cohort in 2018 and 2019 than in 2007, 2010, and 2011 (p < 0.01). The rate of Cx5 did not differ by year in the clinical cohort.
Conclusions: The rates of complications, hospitalisations, and ER visits did not increase among these patients. Oral quinolone combined with intravenous aztreonam reduced the rate of febrile complications compared to quinolone only and is safe for use after a prostate biopsy.
Source of Funding: none

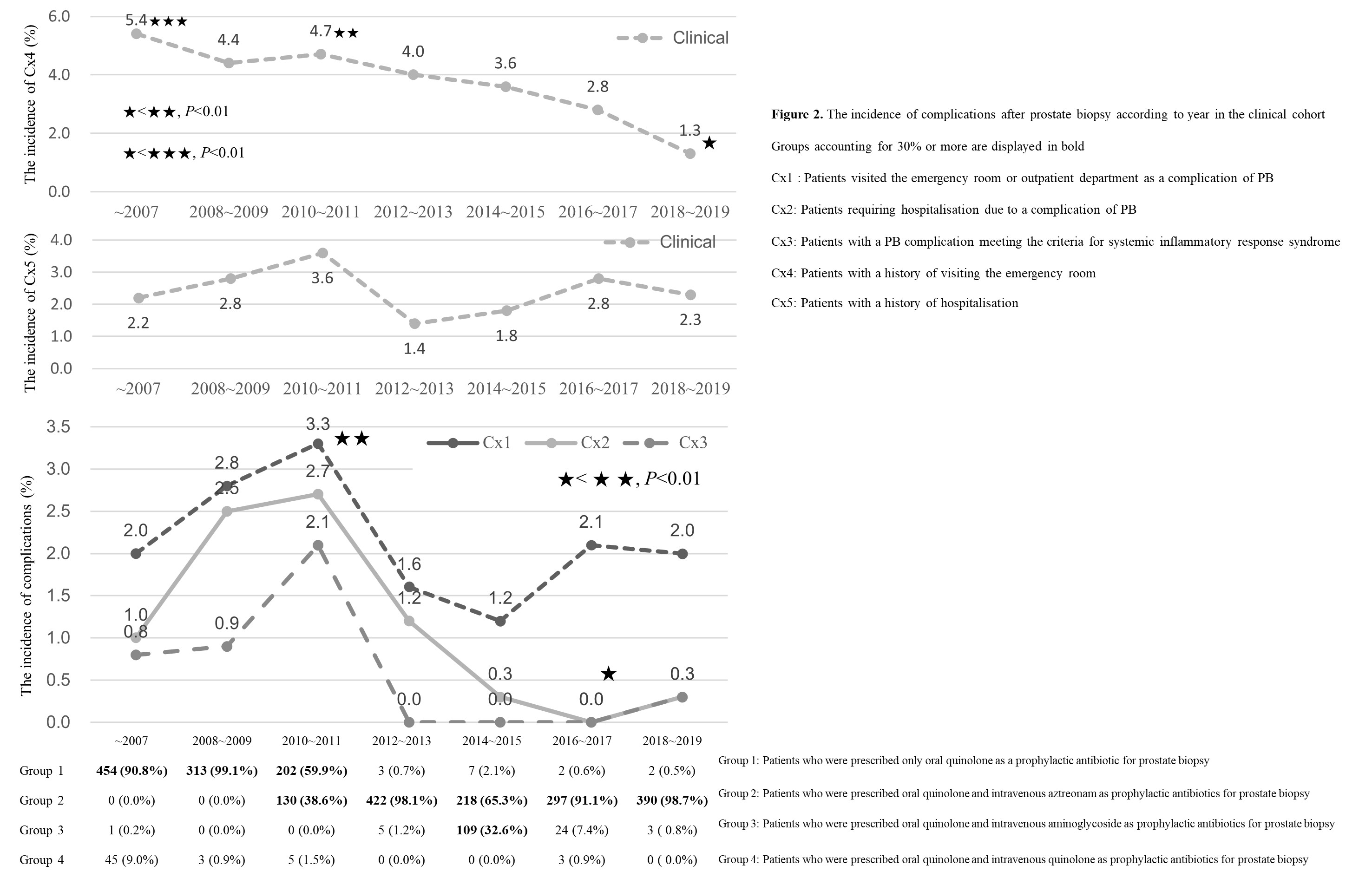
Methods: Clinical data were obtained from 3,638 patients who underwent PB at our hospital from 1997 to 2019. We investigated the complication rates according to group of antibiotic regimens (Figure1). To analyse the incidence of complications according to the type of antibiotic prescribed, patients who experienced procedure-related complications within 2 weeks of the PB and were treated in the ER or in an outpatient setting were categorised as Cx1(complication1). Other definition of complication outcomes are described in Figure2.
Results: The clinical cohort in this study included 2,638 patients; 1,457 of these were administered oral quinolone and IV aztreonam. The rate of complications was significantly lower in patients who received oral quinolone and IV aztreonam than in those who received oral quinolone only (p=0.004). Two (0.1%) patients experienced febrile complications. The rate of Cx4 was significantly lower in the clinical cohort in 2018 and 2019 than in 2007, 2010, and 2011 (p < 0.01). The rate of Cx5 did not differ by year in the clinical cohort. The rate of Cx4 was significantly lower in the clinical cohort in 2018 and 2019 than in 2007, 2010, and 2011 (p < 0.01). The rate of Cx5 did not differ by year in the clinical cohort.
Conclusions: The rates of complications, hospitalisations, and ER visits did not increase among these patients. Oral quinolone combined with intravenous aztreonam reduced the rate of febrile complications compared to quinolone only and is safe for use after a prostate biopsy.
Source of Funding: none



.jpg)
.jpg)