Back
Poster, Podium & Video Sessions
Moderated Poster
MP54: Bladder Cancer: Non-invasive II
MP54-10: Utility of a Urine-Based DNA Methylation Test for Surveillance in Non-muscle Invasive Bladder Cancer: A Pilot Study
Monday, May 16, 2022
8:45 AM – 10:00 AM
Location: Room 228
Seyedeh Sanam Ladi Seyedian*, Alireza Ghoreifi, Sidney Roberts, Farshad Sheybaee Moghadam, Hamed Ahmadi, Los Angeles, CA, Saum Ghodoussipour, New Brunswick, CA, Muhannad Alsyouf, Los Angeles, CA, Paolo Piatti, Benjamin Jara, Lucy Sanossian, Irvine, CA, Sumeet Bhanvadia, Anne Schuckman, Hooman Djaladat, Gangning Liang, Siamak Daneshmand, Los Angeles, CA

Seyedeh Sanam Ladi Seyedian, MD
University of Southern California
Poster Presenter(s)
Introduction: Currently available urine-based tests used for surveillance of non-muscle invasive bladder cancer (NMIBC) are suboptimal. Here, we explore the feasibility of a newly developed urine-based DNA methylation test for detection of recurrence in NMIBC.
Methods: Patients who had evidence of bladder cancer recurrence on surveillance cystoscopy or were referred to our center for blue light transurethral resection of bladder tumor (TURBT) between October 2019 and August 2021 were enrolled. Urine samples were collected before and two months after TURBT and then at each surveillance cystoscopy afterwards. Samples were analyzed with Bladder CARE, a urine-based assay that measures methylation levels of 3 bladder cancer specific biomarkers (TRNA-Cys, SIM2, and NKX1-1) and two internal control loci using methylation-sensitive restriction enzymes coupled with qPCR. Results are reported as Bladder CARE Index (BCI) score and categorized as “positive” (BCI>5) or “negative” (BCI=5). Association between change in BCI score and category with TURBT pathology and recurrence was assessed.
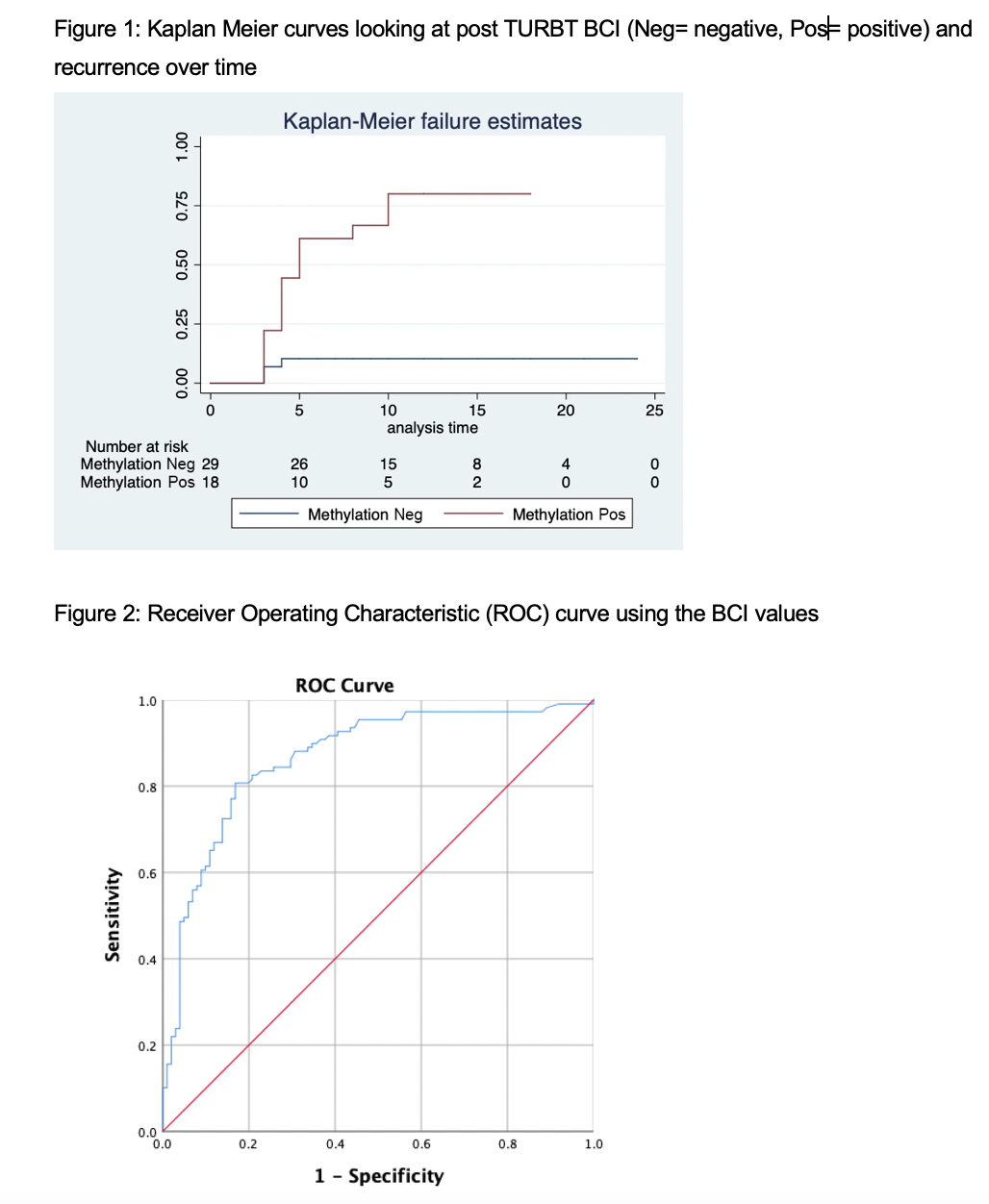
Results: A total of 190 patients with NMIBC were included (median age of 73, 77% male). Of patients under surveillance during the study period, 40/147 (27%) had recurrence, in all but four the BCI became positive prior to or at the time of recurrence. BCI was able to predict the recurrence within a median of 7 months prior. Post-TURBT samples were available in 39/95 (41%) patients. Patients with a positive BCI two months following the TURBT had significantly lower recurrence free survival at 2 years (P= 0.001, Fig. 1). The Receiver Operating Characteristic (ROC) curve demonstrated the sensitivity, specificity, positive predictive values, and negative predictive value of 93%, 65%, 73.5%, and 89.5%, respectively (AUC=0.867, Fig. 2). The false positive and negative rate of Bladder CARE was 35% and 7.3%, respectively. Cytology was only positive or atypical in 27% of the recurrences.
Conclusions: Our preliminary findings suggest a potential utility for this urine-based methylation test in early detection of recurrence in NMIBC. Larger sample size with longer follow-up is required to validate these outcomes.
Source of Funding: Zymo Research Corp, Pangea Laboratory
Methods: Patients who had evidence of bladder cancer recurrence on surveillance cystoscopy or were referred to our center for blue light transurethral resection of bladder tumor (TURBT) between October 2019 and August 2021 were enrolled. Urine samples were collected before and two months after TURBT and then at each surveillance cystoscopy afterwards. Samples were analyzed with Bladder CARE, a urine-based assay that measures methylation levels of 3 bladder cancer specific biomarkers (TRNA-Cys, SIM2, and NKX1-1) and two internal control loci using methylation-sensitive restriction enzymes coupled with qPCR. Results are reported as Bladder CARE Index (BCI) score and categorized as “positive” (BCI>5) or “negative” (BCI=5). Association between change in BCI score and category with TURBT pathology and recurrence was assessed.
Results: A total of 190 patients with NMIBC were included (median age of 73, 77% male). Of patients under surveillance during the study period, 40/147 (27%) had recurrence, in all but four the BCI became positive prior to or at the time of recurrence. BCI was able to predict the recurrence within a median of 7 months prior. Post-TURBT samples were available in 39/95 (41%) patients. Patients with a positive BCI two months following the TURBT had significantly lower recurrence free survival at 2 years (P= 0.001, Fig. 1). The Receiver Operating Characteristic (ROC) curve demonstrated the sensitivity, specificity, positive predictive values, and negative predictive value of 93%, 65%, 73.5%, and 89.5%, respectively (AUC=0.867, Fig. 2). The false positive and negative rate of Bladder CARE was 35% and 7.3%, respectively. Cytology was only positive or atypical in 27% of the recurrences.
Conclusions: Our preliminary findings suggest a potential utility for this urine-based methylation test in early detection of recurrence in NMIBC. Larger sample size with longer follow-up is required to validate these outcomes.
Source of Funding: Zymo Research Corp, Pangea Laboratory


.jpg)
.jpg)