Back
Poster, Podium & Video Sessions
Moderated Poster
MP54: Bladder Cancer: Non-invasive II
MP54-15: A Multicenter Study of 2-year Outcomes Following Hyperthermia Therapy with Mitomycin C in Treating BCG Unresponsive Non-Muscle Invasive Bladder Cancer: Recirculant Hyperthermic Intravesical Chemotherapy
Monday, May 16, 2022
8:45 AM – 10:00 AM
Location: Room 228
Andrew Stemberger*, Wei Phin Tan, Camden, NJ, Ana Plata Bello, Juan Carlos Garcia Alvarez, Canary Islands, Spain, Félix Guerrero-Ramos, Daniel A. González-Padilla, Madrid, Spain, Cajetan Nzeh, Gladbeck, Germany, Jose Manuel de la Morena, Ignacio Gonzalez Valcarcel de Torres, Madrid, Spain, Kees Hendricksen, Amsterdam, Netherlands, Francisco Javier Díaz-Goizueta, J. Fernandez Del Alamo, Madrid, Spain, Francesco Chiancone, Paolo Fedelini, Naples, Italy, Massimiliano Poggio, Francesco Porpiglia, Turin, Italy, Victoria C. Gonzalo Rodríguez, Javier Montero Torres, Burgos, Spain, Daniel Wilby, Richard Robinson, Portsmouth, United Kingdom, Alejandro Sousa-Escandón, Juan León-Mata, Lugo, Spain, Jose L Pontones Moreno, Francisco Delgado Molina, Valencia, Spain, Miguel A. Adriazola Semino, Palencia, Spain, Jesús Calleja-Escudero, Valladolid, Spain, Joan Palou Redorta, Barcelona, Spain, Wei Shen Tan, London, United Kingdom

Andrew Stemberger, BS
Cooper Medical School of Rowan University
Poster Presenter(s)
Introduction: Bacillus Calmette–Guérin (BCG) unresponsive non-muscle invasive bladder cancer (NMIBC) is a challenging disease to manage. We aim to evaluate the recurrence-free survival (RFS) and progression free survival (PFS) of patients treated with chemo hyperthermia (CHT) with mitomycin C using the Combat bladder recirculation system.
Methods: We retrospectively evaluated the Hyperthermic Chemotherapy registry - a prospective, multi-institutional registry of 1,028 patients with NMIBC, confirmed on transurethral resection of bladder tumor, who underwent CHT between 2012 and 2020. Mitomycin C is heated to 43°C using an aluminum heat exchanger that enables efficient heat transfer and accurate temperature control within ±0.5 °C.
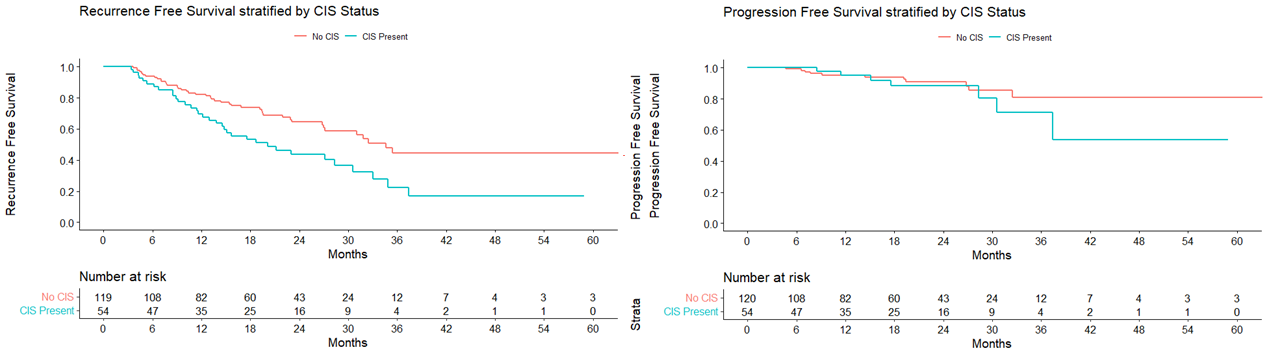
Results: There was a total of 174 were patients who had BCG unresponsive disease and were treated with CHT with Mitomycin C. Median follow up was 16.8 months (Interquartile range (IQR): 9.1 – 26.0). Median age was 68.9 years (IQR: 59.8 – 76.2). A total of 141 (81.0%) patients were men and 33 (19.0%) were women. RFS at 12 and 24 months were 78.1% (95% CI 72% – 84.7%) and 57.4% (95% CI 49.7% – 66.3%), respectively. The PFS at 12 months and 24 months were 95.2% (95% CI 91.8% – 98.7%) and 90.1% (95% CI 84.8% and 95.8%), respectively. On subgroup analysis of the BCG unresponsive cohort by the presence of CIS, the RFS at 12 months was 69.5% (95% CI 58.1% – 83.2%) in the CIS positive with/without papillary group and 82.2% (95% CI 75.4% – 89.6%) in the CIS negative with papillary only group. The RFS at 24 months was 43.6% (95% CI 31.4% – 60.4%) in the CIS positive with/without papillary group and 64.5% (95% CI 55.4% – 75.1%) CIS negative with papillary only group. Minor adverse events occurred in 33 (19.2%) of patients and severe events occurred in 2 (1.1%).
Conclusions: CHT with MMC using the Combat BRS is effective in the medium term and has a favorable adverse event profile in BCG unresponsive patients. This may represent an attractive treatment option in a space where there are limited treatment options available.
Source of Funding: Registry maintained by Combat Medical.
Methods: We retrospectively evaluated the Hyperthermic Chemotherapy registry - a prospective, multi-institutional registry of 1,028 patients with NMIBC, confirmed on transurethral resection of bladder tumor, who underwent CHT between 2012 and 2020. Mitomycin C is heated to 43°C using an aluminum heat exchanger that enables efficient heat transfer and accurate temperature control within ±0.5 °C.
Results: There was a total of 174 were patients who had BCG unresponsive disease and were treated with CHT with Mitomycin C. Median follow up was 16.8 months (Interquartile range (IQR): 9.1 – 26.0). Median age was 68.9 years (IQR: 59.8 – 76.2). A total of 141 (81.0%) patients were men and 33 (19.0%) were women. RFS at 12 and 24 months were 78.1% (95% CI 72% – 84.7%) and 57.4% (95% CI 49.7% – 66.3%), respectively. The PFS at 12 months and 24 months were 95.2% (95% CI 91.8% – 98.7%) and 90.1% (95% CI 84.8% and 95.8%), respectively. On subgroup analysis of the BCG unresponsive cohort by the presence of CIS, the RFS at 12 months was 69.5% (95% CI 58.1% – 83.2%) in the CIS positive with/without papillary group and 82.2% (95% CI 75.4% – 89.6%) in the CIS negative with papillary only group. The RFS at 24 months was 43.6% (95% CI 31.4% – 60.4%) in the CIS positive with/without papillary group and 64.5% (95% CI 55.4% – 75.1%) CIS negative with papillary only group. Minor adverse events occurred in 33 (19.2%) of patients and severe events occurred in 2 (1.1%).
Conclusions: CHT with MMC using the Combat BRS is effective in the medium term and has a favorable adverse event profile in BCG unresponsive patients. This may represent an attractive treatment option in a space where there are limited treatment options available.
Source of Funding: Registry maintained by Combat Medical.


.jpg)
.jpg)