Back
Poster, Podium & Video Sessions
Moderated Poster
MP54: Bladder Cancer: Non-invasive II
MP54-19: Protein Disulfide Isomerase as a Predictive Biomarker for Non-muscle Invasive Bladder Cancer Recurrence
Monday, May 16, 2022
8:45 AM – 10:00 AM
Location: Room 228
Kit L. Yuen*, Chia-Hao Wu, Christopher R. Silvers, Alexis Steinmetz, Hiroshi Miyamoto, Edward M. Messing, Yi-Fen Lee, Rochester, NY

Kit Yuen, MD, MA
University of Rochester Medical Center
Poster Presenter(s)
Introduction: High recurrence rates found in non-muscle invasive bladder cancer (NMIBC) pose a financial and psychological burden for patients. We demonstrated that bladder cancer (BC) cells release protein disulfide isomerase (PDI), a redox-regulated ER-resident protein, via extracellular vesicles (EVs) when experiencing high levels of ER stress for their own survival. Chronic exposure to PDI-enriched BC EVs induced malignant transformation in recipient non-transformed cells. We hypothesized that the dynamic redox state in BC cells determines PDI cellular localization and tumors with elevated PDI preferentially incorporate PDI into EVs for removal. Furthermore, PDI expression levels and subcellular localization in tumors from NMIBC patients may predict NMIBC recurrence.
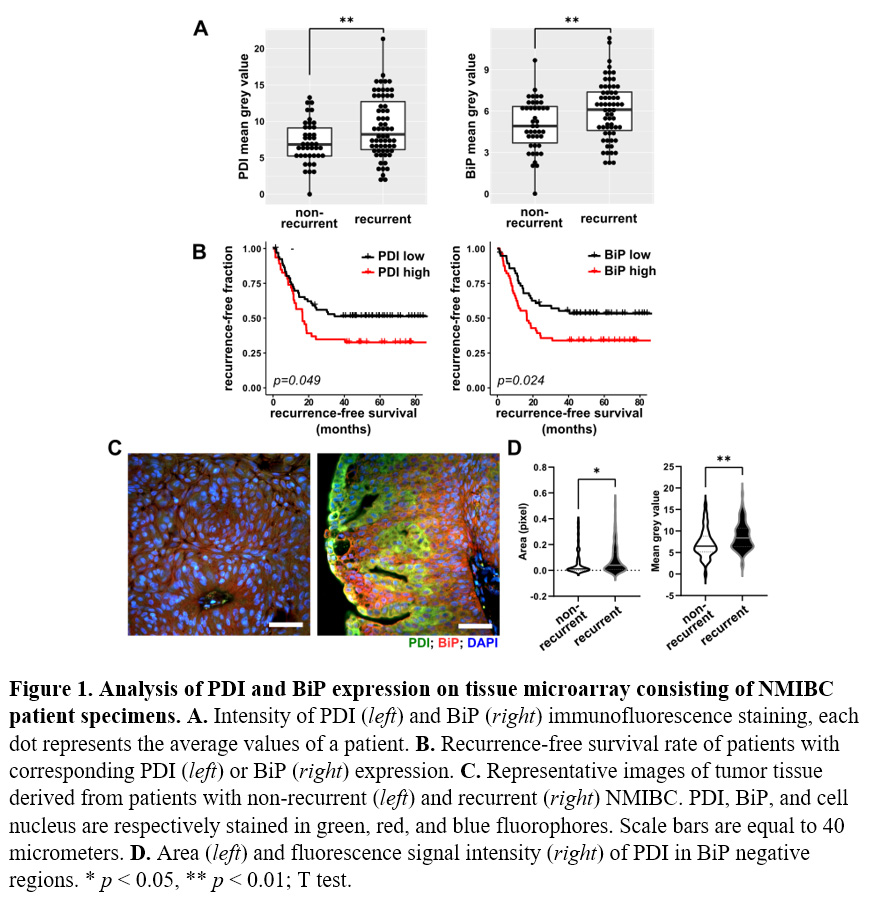
Methods: Retrospective chart review identified 122 patients with newly diagnosed low-grade NMIBC classified into 2 groups: recurrent (N=55) and non-recurrent (N=67) during follow-up period. Formalin fixed paraffin embedded tumor specimens from index transurethral resection of bladder tumor (TURBT) were obtained and a tissue microarray (TMA) was constructed. Immunofluorescent staining of TMA sections was conducted using antibodies against PDI and BiP, an ER stress marker. Stained tissues were photographed and tumor regions in each field were manually masked and confirmed by a genitourinary pathologist. Within each tumor region, total antibody labeling was determined by measuring the mean pixel intensity values with NIH ImageJ/Fiji. Manders coefficient was used to assess the colocalization of epitopes using JACoP plugin in ImageJ.
Results: Tumor tissues from patients that recurred expressed significantly higher levels of PDI and BiP. Moreover, high PDI and BiP expression predicted worse recurrence-free survival (Fig 1A,B). These data suggest that tissues under higher levels of ER stress—as indicated by higher levels of PDI and BiP expression—have a higher risk of recurrence. Significantly higher levels of PDI were detected in non-ER cytosol in recurrent cohort tumors (Fig 1D).
Conclusions: This study provides a novel mechanism for BC recurrence and valuable clinical predictive biomarkers for NMIBC recurrence and progression. Future clinical applications targeting this pathway may be an avenue to prevent NMIBC recurrence.
Source of Funding: NCI R01 CA173
Methods: Retrospective chart review identified 122 patients with newly diagnosed low-grade NMIBC classified into 2 groups: recurrent (N=55) and non-recurrent (N=67) during follow-up period. Formalin fixed paraffin embedded tumor specimens from index transurethral resection of bladder tumor (TURBT) were obtained and a tissue microarray (TMA) was constructed. Immunofluorescent staining of TMA sections was conducted using antibodies against PDI and BiP, an ER stress marker. Stained tissues were photographed and tumor regions in each field were manually masked and confirmed by a genitourinary pathologist. Within each tumor region, total antibody labeling was determined by measuring the mean pixel intensity values with NIH ImageJ/Fiji. Manders coefficient was used to assess the colocalization of epitopes using JACoP plugin in ImageJ.
Results: Tumor tissues from patients that recurred expressed significantly higher levels of PDI and BiP. Moreover, high PDI and BiP expression predicted worse recurrence-free survival (Fig 1A,B). These data suggest that tissues under higher levels of ER stress—as indicated by higher levels of PDI and BiP expression—have a higher risk of recurrence. Significantly higher levels of PDI were detected in non-ER cytosol in recurrent cohort tumors (Fig 1D).
Conclusions: This study provides a novel mechanism for BC recurrence and valuable clinical predictive biomarkers for NMIBC recurrence and progression. Future clinical applications targeting this pathway may be an avenue to prevent NMIBC recurrence.
Source of Funding: NCI R01 CA173


.jpg)
.jpg)